Interview Summary
IMROZ represents a landmark study in multiple myeloma and is the first global Phase III study of an anti-CD38 monoclonal antibody (mAb) in combination with standard-of-care bortezomib, lenalidomide, and dexamethasone (VRd) to show a significant improvement in progression-free survival (PFS), together with deep and sustained responses, in newly-diagnosed patients not intended for transplant. During a joint interview conducted by the European Medical Journal (EMJ), two leading experts in the field of myeloma and co-investigators on the IMROZ study, Meral Beksac from the Division of Haematology, Ankara Liv Hospital Istinye University, Türkiye; and Mohamad Mohty from Sorbonne University, Saint-Antoine Hospital, Paris, France, discussed the findings and clinical implications of this important study of the isatuximab (Isa) plus VRd regimen. Beksac and Mohty reviewed the methodology and key efficacy and safety data from the IMROZ trial, and offered their perspectives on evaluating patient eligibility for haematopoietic stem cell transplantation (HSCT). The experts also considered the clinical impact of IMROZ on the management of newly diagnosed myeloma, and the potential positioning of quadruplet regimens such as Isa-VRd as the new first-choice frontline treatment for transplant-ineligible patients.Background and Methodology of the IMROZ Study
Despite what Mohty described as “progressive advances in the field” over recent years, significant unmet needs remain in the front-line management of multiple myeloma, particularly in those patients ineligible for HSCT.1-3 “Myeloma is a disease of the elderly, and many patients are frail, and have comorbidities. The median age in Western countries and North America is 70 or above,” Mohty explained.1,2 “In many countries, the standard of care in this population is the VRd combination, which was approved and validated, thanks to the SWOG SO777 randomised trial.”4 This first line of treatment is particularly important for older patients with newly diagnosed multiple myeloma (NDMM), who derive the most benefit when efficacious regimens are used early, given that some do not have the chance to receive any subsequent lines of therapy.5,6
Outlining the rationale for the IMROZ trial, Mohty described this as: “A smart trial which took the standard of care VRd, probably the most popular regimen at the time of initiation of this trial in 2017, and said, ‘Can we add to VRd an antiCD38 mAb, namely isatuximab, and see whether this quadruplet, Isa-VRd, can do better than VRd’ which is well established, safe, and easy to use?” This global, randomised, Phase III, open-label study enrolled patients between December 2017–March 2019 across 21 different countries.5,6 Patients with NDMM not intended for transplant were randomised 3:2 to receive Isa-VRd induction followed by Isa-Rd continuous therapy, or VRd induction followed by Rd continuous therapy (Figure 1).5,6
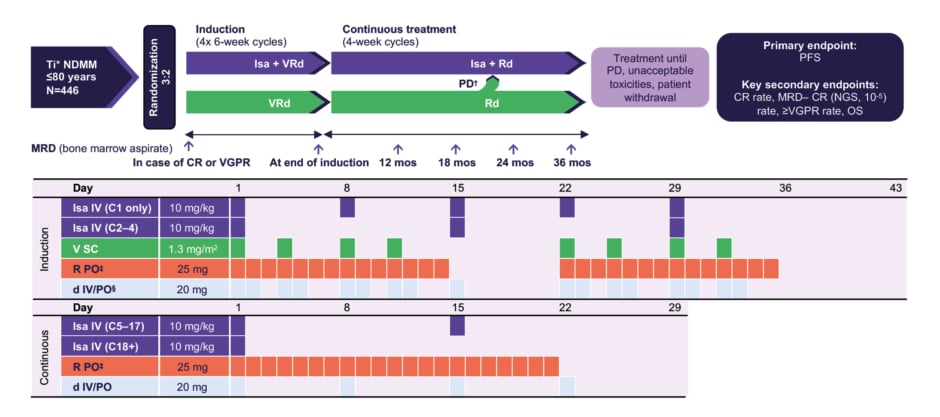
Figure 1: IMROZ study design.5
*Patients considered Ti due to age or comorbidities.
?In the continuous phase, patients randomised to the VRd arm who experience PD may cross over to receive Isa-Rd.
‡10 mg/day if eGFR 30–<60 mL/min/1.73 m2.
§If aged ≥75 years, d was administered on days 1, 4, 8, 11, 15, 22, 25, 29, and 32.
C: cycle; d: dexamethasone; CR: complete response; eGFR: estimated glomerular filtration rate; Isa: isatuximab; mos: months; MRD–: minimal residual disease negativity; NDMM: newly diagnosed multiple myeloma; NGS: next generation sequencing; OS: overall survival; PD: progressive disease; PFS: progression-free survival; PO: orally; R: lenalidomide; SC: subcutaneous; Ti: treatment ineligible; V: bortezomib; Vrd: bortezomib, lenalidomide, dexamethasone; VGPR: very good partial response.
The Transplant-Ineligible Patient Population
Experts agreed that the patient population of the IMROZ study was broadly typical of transplant-ineligible NDMM patients encountered in the clinical setting. As Beksac outlined: “The age profile for this study ranged between 55–80 years. Around a third of patients were aged between 75–80 years, which is important to mention, as this covers a population which we frequently see in the clinic.”5,6 Mohty added: “If you ask me to describe the patient population of the IMROZ study, and whether it is typical of transplant-ineligible NDMM patients encountered in the clinical setting, my answer is yes and no. Although it is an elderly population, by definition it excluded patients who are very old (above 80 years) as well as those with comorbidities or poor performance status. That is not a bad thing because it aligns with the demographic trend in Western countries where patients are growing old but remain fit.”
Beksac also confirmed that the patient profile in IMROZ was representative of other clinical characteristics, such as International Staging System (ISS) score, performance status, and renal function. “There was good representation of a high-risk patient profile in IMROZ […] which is important for isatuximab because earlier studies have shown data in favour of even better efficacy in this specific patient cytogenic profile,” she noted.
In terms of factors currently used to determine patients’ suitability to proceed down the path to HSCT, experts pointed to differences between Europe and the USA. “The cut-off for transplant eligibility differs from one country to another, and today we do not have consensus around an agreed upon age limit. We further rely on frailty and the patient’s performance status, not only at diagnosis but at later stages as well,” explained Beksac. She also noted: “The trend in receiving transplant is now decreasing over time. The introduction of very effective systemic regimens with similar efficacy to HSCT has seen a shift towards non-transplant regimens, particularly in the USA.”
Mohty agreed that the IMROZ study could have important implications when evaluating patients’ transplant eligibility. “In many centres in Europe, these patients would be considered transplant eligible. Hence for this group of patients who are not too old, but not too young, and fit, IMROZ is offering a non-transplant-based treatment with similar efficacy to what can be achieved with HSCT,” he remarked.
Experts also provided some personal perspectives on factors currently used to assess transplant eligibility, highlighting patient perceptions and willingness to undergo the procedure as important considerations. “Transplant requires hospitalisation and is associated with mucositis, pain, diarrhoea, aplasia, and infections. Patients tend to ask ‘Doctor Google’ about the potential side effects they face, and may then be terrified into requesting a non-transplant approach,” Mohty elaborated. “In the ideal world, we would like to listen to and follow patient preference,” Mohty continued; however, ‘real-world’ factors such as cost and access must also be considered.
Beksac also stressed that transplant eligibility should not be defined purely by age, but confirmed that “the upper age limit in the IMROZ study has value in terms of demonstrating the tolerability of quadruplet treatment in such a population.”
Key Efficacy Results from IMROZ
According to the IMROZ study findings presented at ASCO, at median 5 years follow-up, the addition of Isa to VRd resulted in a statistically significant reduction in the risk of progression or death by 40.4% (hazard ratio [HR]: 0.596; p=0.0005), with the median PFS not reached in the Isa-VRd arm versus 54.34 in the VRd arm (Figure 2).5,6 The 60-month PFS rate was 63.2% versus 45.2% with Isa-VRd and VRd, respectively. Furthermore, a consistent benefit was observed with the quadruplet Isa-VRd regimen across all patient subgroups, including difficult-to-treat patient populations with negative prognostic factors.5,6 Describing these results from the IMROZ study as “impressive”, Mohty explained: “It was expected that Isa-VRd would be better than VRd. However, I think the big surprise from this trial was the magnitude of superiority. From the first analysis, it looks like just by adding Isa, an anti-C38 MAb, to the triplet VRd standard of care, you achieve an improvement of 40%, which for me was totally unexpected.”
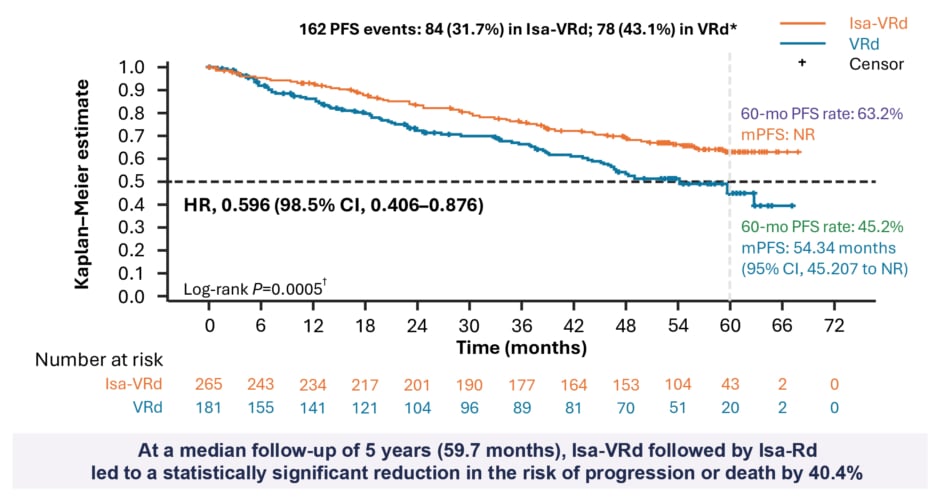
Figure 2: IMROZ study primary endpoint results: progression-free survival (intention to treat population).5
*Cutoff date for PFS analysis: 26 September, 2023 (median follow-up: ~5 years).
?Nominal one-sided P value.
HR: hazard ratio; Isa: isatuximab; ITT: intention to treat; mPFS: median PFS; NR: not reached; PFS: progression-free survival; VRd: bortezomib, lenalidomide, dexamethasone.
Beksac agreed with Mohty that the PFS benefit demonstrated by Isa-VRd in this study was outstanding, and suggested that the audience would be inclined to compare results from IMROZ to the MAIA trial of daratumumab (dara) plus Rd in untreated myeloma.7 “I think we can easily say that Isa-VRd is more effective than Dara-Rd was in the MAIA protocol, and we owe this to the different drug partners in the regimen,” she explained.
In the IMROZ study, Isa-VRd also produced deep response rates compared to VRd, with a statistically significant improvement in the minimal residual disease (MRD) negative complete response (CR) rate, as well as higher rates of MRD, and almost double sustained MRD, for ≥12 months at any time in the intention to treat population (Figure 3).5,6 Commenting on these results, Beksac noted: “There was a substantial depth of response with Isa-VRd, as seen directly in the MRD rates. There was also a very clear difference between the PFS curves in the control arm versus the treatment arm in IMROZ.” On the comparison with MAIA, she added: “the biggest difference between the two studies is the depth of response as measured by MRD. This may be a signal for the future, that we may be able to de-escalate treatment.”
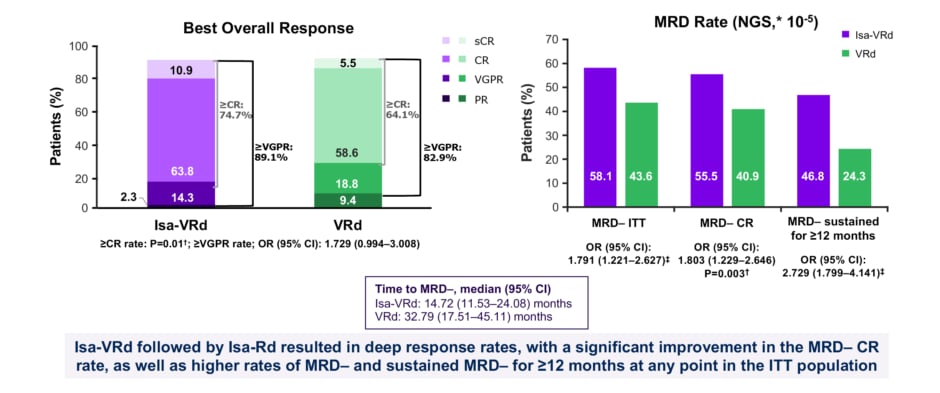
Figure 3: IMROZ study: depth of response.5
*Adaptive Biotechnologies clonoSEQ.
?Stratified Cochran-Mantel-Haenszel test. Two-sided significance level is 0.025.
‡P value not reported; not a key secondary endpoint.
CR: complete response; Isa: isatuximab; ITT: intention to treat; MRD–: minimal residual disease negativity; NGS: next generation sequencing; OR: odds ratio; VGPR: very good partial response; VRd: bortezomib, lenalidomide, dexamethasone.
As further useful context for the IMROZ study, the experts highlighted the results of the BENEFIT trial, also disclosed at ASCO 2024, which enables the value of both bortezomib and the anti-CD38 mAb within the regimen to be clearly elucidated.8 Beksac commented that, collectively, these trials “show the relative impact of using a quadruplet, a triplet, or a less intensive regimen.” “The more drugs we use, the deeper the responses are; so long as patients tolerate it,” she confirmed.
Key Safety Results from IMROZ
In the IMROZ study, Isa-VRd proved to be well-tolerated in NDMM patients not intended for transplant, and the safety profile remained consistent with that for each individual agent.5,6 “I would summarise it simply as there was nothing new under the sun,” confirmed Mohty. “Isa-VRd did not prove to be significantly more toxic than VRd alone, which is in line with the safety profile of isatuximab.” He explained that this favourable safety profile was anticipated from the outset of the trial because “at the end of day, when using the same drugs, same families, same combinations, then you can roughly expect what you’re going to see.” He added: “VRd and isatuximab are both very well known, so hundreds, if not thousands, of patients have received these agents.”
Emphasising the tolerability of this quadruplet regimen, Beksac made the point that the IMROZ study evaluated “a real VRd regimen and not a ‘VRd-light’ version with dose reductions for the elderly.” Rates of serious treatment-emergent adverse events (TEAE) and TEAEs leading to definitive discontinuation were also similar between treatment arms in the study.5,6 In particular, Beksac highlighted the low discontinuation rate due to AEs among the IMROZ population (22.8% with Isa-VRd versus 26.0% with VRd) as further evidence of the quadruplet’s favourable safety profile.6 However, Mohty did note that demonstrably frail patients were excluded from the IMROZ study, which is a factor to consider in the clinical practice setting. “At some point, people will forget the inclusion criteria and say elderly patients can receive quadruplet Isa-VRd. That’s not exactly the case,” he cautioned, “if you are elderly and frail, in my opinion, the regimen will not be so well tolerated.”
Looking at specific AEs, Beksac drew comparisons to the PERSEUS trial, which evaluated the dara plus VRd quadruplet in the transplant-eligible setting.9 “In PERSEUS, the most striking side effects were infections, and the percentages seen in the PERSEUS study are very similar to what we are seeing now with the IMROZ study. I think this is a good comparison, even though the age groups and mAb are different,” she noted.
Integrating IMROZ Findings into Daily Clinical Practice
IMROZ is a registrational study intended to support USA and EU regulatory approval of the Isa-VRd regimen for the treatment of transplant-ineligible NDMM. The FDA has recently granted Isa-VRd Priority Review in this setting based on the results of the IMROZ study, with a target action date set for September 2024.10 Overall, experts agreed that the unparalleled improvement in PFS seen in the IMROZ study, with consistent and profound responses, supports the frontline use of Isa-VRd as a new standard of care in NDMM patients not intended for transplant.5,6
When asked whether quadruplet regimens such as Isa-VRd will now be the first choice in transplant-ineligible NDMM over standard triplets, Mohty replied that “the short answer is yes.” He continued: “Based on this trial, quadruplet should become the preferred regimen in the population of patients represented by these inclusion criteria.” Beksac agreed that Isa-VRd would now play a role in the front-line management of transplant-ineligible MM. “My interpretation would be that this protocol will be conceived as an applicable protocol that can be adapted to our daily practice,” she commented. Mohty suggested that, based on trial results such as IMROZ, the “story” in myeloma was now changing and evolving. “It’s not transplant versus no transplant, it’s who is going to receive quadruplet and who’s going to receive less than a quadruplet; who is fit and who is unfit,” he explained.
Both experts indicated that physicians would likely adapt the Isa-VRd regimen for real-world use, and Mohty explained that this would be based on clinical judgement. “People will try to do some interpretation on all this,” he elaborated. “They may say that it is licensed up to the age of 80 years, but I am going to adjust the dose for my 82-year-old patient.” This point was echoed by Beksac: “I think we will adapt this regimen. Most probably we will combine the quadruplet in a kind of VRd-light version to start with, and maybe we can adapt later depending on the tolerability of the patient.”
Mohty also suggested: “Based on this trial, we will likely see a decline in the number of transplants. With the IMROZ data, we have something valid that we can offer patients without any prejudice to their outcome.” However, he cautioned that the alternative to transplant consists of continuous treatment for life, and added: “Given the number of months’ survival that is achieved with Isa-VRd, patients will be taking treatment for a long time.” This point was reiterated by Beksac who explained: “Some patients who are active and do not want to stay off work for a couple of months over the transplant period may instead choose a long-term treatment versus a stem cell protocol.”
Looking to the future, Beksac also noted that the enhanced efficacy of the Isa-VRd regimen has the potential to meet individual patient needs, irrespective of transplant eligibility status. “There is also the potential that this protocol may move forward to younger patients who are not willing to undergo transplant, and who want to have an intensive regimen,” she suggested. Mohty agreed: “It looks like if you are relatively old and fit you can do very well with Isa-VRd, and that is going to be your new best treatment option.”
Overall, the impressive efficacy results from the IMROZ trial are “good news” for the myeloma community, Mohty reiterated. “Until recently, we thought that transplant is the gold standard for everybody whenever possible. Now, we have a more nuanced answer, offering a regimen that actually is as effective, and may even be better, than transplant. So, it’s a most welcome addition to what we do,” he concluded.






