This article has been developed and funded by Jazz Pharmaceuticals.
For many patients diagnosed with acute myeloid leukaemia (AML), long-term survival remains elusive.1 For the approximately one third of patients who fall into high-risk categories (defined as therapy-related AML or AML with myelodysplasia-related changes), the outlook is particularly poor.2-4 Compared with other AML subtypes, high-risk AML is associated with lower remission rates and shorter overall survival (OS) following treatment with conventional chemotherapy.5
Despite their poor prognosis, many patients with AML stay hopeful for life-extending treatment.5,6 Intensive chemotherapy followed by haematopoietic stem cell transplantation (HSCT) remains a key approach to reaching that goal.7,8 Indeed, the European LeukemiaNet (ELN) guidelines suggest that patients’ initial assessment should be focused on their eligibility for intensive induction chemotherapy.9 They also state that age alone should not be the principal factor guiding therapy, with some randomised trials suggesting that older, medically fit patients may benefit more from ‘intensive’ rather than ‘non-intensive’ induction therapy.9
Vyxeos Liposomal Optimises the Delivery of Daunorubicin and Cytarabine to Significantly Improve Survival in High-Risk Acute Myeloid Leukaemia Versus Conventional Chemotherapy10,11
Until recently, conventional chemotherapy regimens, a ‘7+3’ combination of cytarabine and daunorubicin, had remained the standard of care for decades.11,12 Today, Vyxeos Liposomal (generic name: daunorubicin 44 mg/cytarabine 100 mg powder for concentrate for solution for infusion [CPX-351]) offers improved outcomes for patients with high-risk AML.11,13
Indicated for the treatment of adults with newly diagnosed therapy-related AML or AML with myelodysplasia-related changes, Vyxeos Liposomal is an advanced, dual-drug liposomal formulation of a synergistic 1:5 molar drug ratio of daunorubicin and cytarabine.10-13 In its pivotal Phase III study, Vyxeos Liposomal became the first chemotherapy in over 40 years to improve survival for patients with high-risk AML compared with conventional chemotherapy (Table 1).11,12

Table 1: Phase III primary endpoint. Vyxeos Liposomal achieved superior OS in patients with high-risk AML versus conventional chemotherapy.11,13
Median follow-up: 20.7 months.11
HR: 0.69; 95% CI: 0.52-0.90; 2-sided p=0.005.13
AML: acute myeloid leukaemia; CI: confidence interval; HR: hazard ratio: OS: overall survival.
Vyxeos Liposomal May Provide a First Step Toward Long-Term Disease Control14
To this day, the first step towards long-term disease control in AML remains the same: reducing the leukaemic burden by inducing complete remission (CR).7,8
In its Phase III study, Vyxeos Liposomal produced a higher rate of CR compared with conventional chemotherapy (37.3% versus 25.6%; 2-sided p=0.040).11 The overall remission rate (CR + CR with incomplete neutrophil or platelet recovery [CRi]) was also higher with Vyxeos Liposomal (47.7% versus 33.3% with conventional chemotherapy; 2-sided p=0.016).11 Additionally, Vyxeos Liposomal achieved a greater rate of CR than conventional chemotherapy achieved for both CR and CRi combined.11
CR may mark the first milestone towards long-term survival.7,8 Once CR is reached, the option of HSCT may be on the horizon for appropriate patients.7 In AML, HSCT is recommended as a post-remission therapy to prolong remission and prevent relapse.15 In the Phase III study, a larger proportion of patients proceeded to HSCT with Vyxeos Liposomal compared with conventional chemotherapy (34% [n=52/153] versus 25% [n=39/156]; 2-sided p=0.098).11
Vyxeos Liposomal Increased Overall Survival Without Increasing the Toxicity Burden Compared with Conventional Chemotherapy11
In the Phase III study, the safety profiles of Vyxeos Liposomal and conventional chemotherapy were comparable for the types, frequency, and severity of adverse events.11 The most frequently reported Grade 3 to 5 adverse events in the Vyxeos Liposomal and conventional chemotherapy cohorts were febrile neutropaenia (68.0% versus 70.9%), pneumonia (19.6% versus 14.6%), and hypoxia (13.1% versus 15.2%).11
While the proportions of patients experiencing adverse events were comparable, patients receiving Vyxeos Liposomal had a longer median treatment phase than those receiving conventional chemotherapy (62 days versus 41 days).11 To normalise this effect, the rate of adverse events per patient year was evaluated.11 Vyxeos Liposomal was associated with a lower median rate of adverse events per patient year than conventional chemotherapy (75.68 versus 87.22).11
Early mortality rates with Vyxeos Liposomal and conventional chemotherapy were 5.9% and 10.6% through Day 30 (2-sided p=0.149), and 13.7% and 21.2% through Day 60 (2-sided p=0.097), respectively.11 Among patients who achieved CR or CRi after initial induction, the median time to neutrophil and platelet recovery was longer with Vyxeos Liposomal (35.0 and 36.5 days, respectively) versus conventional chemotherapy (29.0 and 29.0 days, respectively).11 As a result, patients receiving Vyxeos Liposomal may require additional monitoring for prolonged thrombocytopenias and neutropenias.11,13 Please refer to the Summary of Product Characteristics for full safety and tolerability information.
The Overall Survival Benefit of Vyxeos Liposomal Over Conventional Chemotherapy is Maintained into the Longer Term14
The results of a prospectively planned, 5-year follow-up OS analysis of the intention-to-treat population in the Phase III study demonstrated that Vyxeos Liposomal may contribute to long-term remission and survival in patients with high-risk AML.14 Most notably, the 5-year Kaplan–Meier-estimated survival rate with Vyxeos Liposomal was more than double that of conventional chemotherapy (18.0% versus 8.0%).14
In a post-hoc analysis of the 5-year data, Vyxeos Liposomal not only improved remission rates in patients with high-risk AML, but also the survival outcomes among those who achieved CR or CRi versus conventional chemotherapy.14 The median OS of patients who achieved CR or CRi with Vyxeos Liposomal was more than double that of conventional chemotherapy (21.7 months [n=50/73] versus 10.4 months [n=43/52]; hazard ratio: 0.59; 95% confidence interval: 0.39–0.88).14,16
Among patients who received Vyxeos Liposomal and underwent HSCT, the post-hoc analysis also showed that OS was greater than 50% at 5 years post-randomisation.17 Median OS from the date of HSCT was not reached with Vyxeos Liposomal (n=25/53) versus 10.25 months with conventional chemotherapy (n=30/39); hazard ratio: 0.51, 95% confidence interval: 0.28–0.90 (Figure 1).14
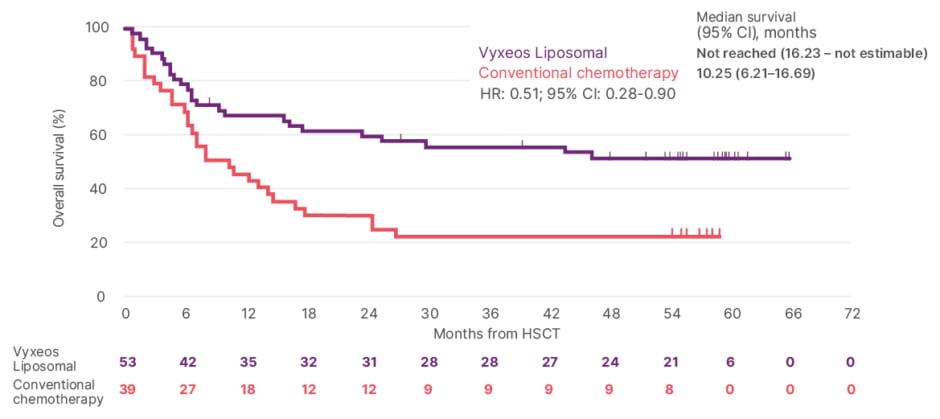
Figure 1: Post-hoc analysis. Overall survival landmarked from date of HSCT.14
5-year estimates were not available as the follow-up time from the date of HSCT was <5 years.14
CI: confidence interval; HR: hazard ratio; HSCT: haematopoietic stem cell transplant.
Adapted from Lancet JE et al.14
Real-World Evidence with Vyxeos Liposomal Reinforces the Results of the Pivotal Phase III Study11,18-20
Adding to the long-term data, the effectiveness and safety of Vyxeos Liposomal have been evaluated in real-world evidence studies from Italy, France, and Germany.18-20 These retrospective, multicentre studies captured data from patients who were diagnosed with high-risk AML and treated with Vyxeos Liposomal in routine clinical practice.18-20
In real-world evidence studies, rather than having strict inclusion and exclusion criteria, all patients must be considered for treatment in clinical practice, including those with comorbidities.21 Reflecting this, the age ranges of patients included in these three real-world studies with Vyxeos Liposomal were wider than those enrolled in the Phase III study.11,18-20 Of note, the authors of the Italian study also concluded that many of the enrolled patients had multiple comorbidities that would have likely precluded their involvement in the Phase III study.18
By analysing the outcomes of a wider breadth of patients, such real-world studies can help paint a more complete picture of a treatment’s safety and efficacy in the post-approval setting.22 With Vyxeos Liposomal, these real-world studies also show that the results of the Phase III study are being reinforced in real-world clinical practice in real-world patients (Table 2).11,18-20 Importantly, the safety profile identified in the Phase III study was also confirmed in all three real-world studies.11,18-20
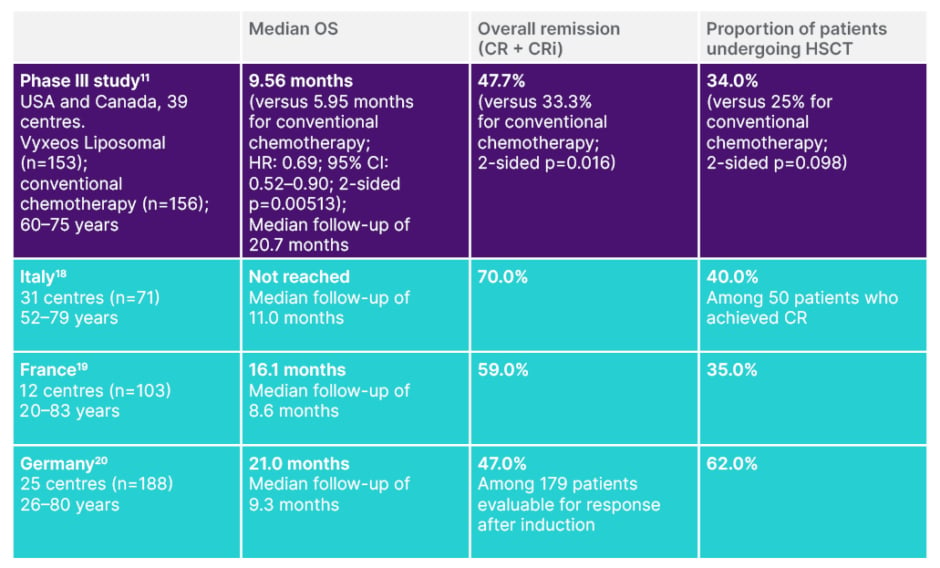
Table 2: Real-world evidence with Vyxeos Liposomal reinforces the results of the Phase III study.11,18-20
Data presented are from studies that have different patient populations, methodologies, and rationales, and are therefore not directly comparable.
CR: complete remission; CRi: complete remission with incomplete neutrophil or platelet recovery; HSCT: haematopoietic stem cell transplantation; OS: overall survival.
Vyxeos Liposomal May be Associated with a Potentially Deeper Response Than Conventional Chemotherapy14,15
These real-world studies also assessed patients’ measurable residual disease (MRD) status.18-20 MRD assessment can be used to detect residual leukaemic cells below the 5% sensitivity of morphologic assessments.23 In patients with AML who achieve remission, MRD assessment can provide powerful prognostic information.15 While data specific to high-risk AML are limited, numerous studies have shown that the presence of MRD in AML is associated with a greater relapse risk and shorter survival compared with MRD negativity.23,24
While MRD was not assessed in the Phase III study, post-hoc analyses of the 5-year data have shown longer median OS among patients achieving remission and in those undergoing HSCT with Vyxeos Liposomal versus conventional chemotherapy.14 This led the study authors to conclude that a potentially deeper response may be possible with Vyxeos Liposomal compared with conventional chemotherapy, and that this hypothesis should be substantiated with MRD analyses in a future clinical trial.14
In these real-world studies, notable proportions of patients treated with Vyxeos Liposomal achieved MRD negativity.18-20 In the Italian study, MRD negative CR was observed in 37.5% (n=15/40) of patients assessed with multiparameter flow cytometry (MFC), and 55.3% (n=21/38) of patients assessed with WT1-based MRD analysis.18
Further, in the French study, among patients who achieved CR or CRi, 28 of 61 (46%) were evaluable for MRD at the time of the first consolidation cycle and were assessed by next-generation sequencing, quantitative PCR, or flow cytometry.19 Among these 28 patients, over half (57%; n=16/28) achieved MRD negativity (defined as <10-3).19
In the German study, among patients with CR or CRi at the time of transplant, routine flow cytometry-based MRD analysis was available for 36 patients.20 Of these 36 patients, 64% (n=23/36) achieved MRD negativity (defined as <10-3).20
Vyxeos Liposomal May Lay the Foundation for Long-Term Survival in High-Risk Acute Myeloid Leukaemia11,14
Intensive chemotherapy remains a starting point that has consistently achieved long-term disease-free survival in AML, the outcome that many patients hope for.6,25 Vyxeos Liposomal offers an intensive treatment for patients with high-risk AML who, until recently, had few options available to them.26 With improved outcomes over conventional chemotherapy, Vyxeos Liposomal may provide a first step toward long-term remission and long-term survival for patients with high-risk AML.11,14
Click here for adverse event reporting details and prescribing information
Explore more data at www.vyxeos.eu
INT-VYX-2200117 Date of preparation: June 2022
References
- Liesveld J. Management of AML: who do we really cure? Leuk Res. 2012;36(12):1475-80.
- Granfeldt Østgård LS et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33(31):3641-9.
- Nagel G et al. Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol. 2017;96(12):1993-2003.
- Arber DA et al. The 2016 revision to the World Health Organisation classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391-405.
- Arber DA, Erba HP. Diagnosis and treatment of patients with acute myeloid leukemia with myelodysplasia-related changes (AML-MRC). Am J Clin Pathol. 2020;154(6):731-41.
- Crawford R et al. Patient-centered insights on treatment decision making and living with acute myeloid leukemia and other hematologic cancers. Patient. 2020;13(1):83-102.
- Hecker J et al. Bridging strategies to allogenic transplant for older AML patients. Cancers (Basel). 2018;10(7):232.
- Stein EM, Tallman MS. Remission induction in acute myeloid leukemia. Int J Hematol. 2012;96(2):164-70.
- Döhner H et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424-47.
- Tolcher AW, Mayer LD. Improving combination cancer therapy: the CombiPlex® development platform. Future Oncol. 2018;14(13):1317-32.
- Lancet JE et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018;36(26):2684-92.
- Talati C, Lancet JE. CPX-351: changing the landscape of treatment for patients with secondary acute myeloid leukemia. Future Oncol. 2018;14(2):1147-54.
- Vyxeos Liposomal. European summary of product characteristics. Available at: https://pp.jazzpharma.com/pi/vyxeos.en.SPC.pdf. Last accessed: 18 July 2022.
- Lancet JE et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2021;8(7):e481-91.
- Lin TL et al. Older adults with newly diagnosed high-risk/secondary AML who achieved remission with CPX-351: phase 3 post hoc analyses. Blood Adv. 2021;5(6):1719-28.
- Supplement to: Lancet JE et al. CPX-351 versus 7+3 cytarabine and daunorubicin chemotherapy in older adults with newly diagnosed high-risk or secondary acute myeloid leukaemia: 5-year results of a randomised, open-label, multicentre, Phase 3 trial. Lancet Haematol. 2021;8(7):e481-91.
- Uy GL et al. Transplant outcomes after CPX-351 vs 7+3 in older adults with newly diagnosed high-risk and/or secondary AML. Blood Adv. 2022;bloodadvances.2021006468.
- Guolo F et al. CPX-351 treatment in secondary acute myeloblastic leukemia is effective and improves the feasibility of allogeneic stem cell transplantation: results of the Italian compassionate use program. Blood Cancer J. 2020;10(10):96.
- Chiche E et al. Real-life experience with CPX-351 and impact on the outcome of high-risk AML patients: a multicentric French cohort. Blood Adv. 2021;5(1):176-84.
- Rautenberg C et al. Real-world experience of CPX-351 as first-line treatment for patients with acute myeloid leukemia. Blood Cancer J. 2021;11(10):164.
- Mahajan R. Real world data: additional source for making clinical decisions. Int J Appl Basic Med Res. 2015;5(2):82.
- London School of Economics (LSE); Gill J et al. The use of real world evidence in the European context: an analysis of key expert opinion. 2016. Available at: https://www.lse.ac.uk/business/consulting/assets/documents/the-use-of-real-world-evidence-in-the-european-context.pdf. Last accessed: 20 May 2022.
- Cluzeau T et al. Measurable residual disease in high-risk acute myeloid leukemia. Cancers (Basel). 2022;14(5):1278.
- Schuurhuis GJ et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD working party. Blood. 2018;131(12):1275-91.
- Rowe JM. Will new agents impact survival in AML? Best Pract Res Clin Haematol. 2019;32(4):101094.
- Drummond MW. Developments in chemotherapy for the treatment of acute myeloid leukemia. Int J Hematol Oncol. 2019;8(2):IJH16.








