Meeting Summary
Prof Salles provided an update on the ongoing first-line follicular lymphoma (FL) studies, demonstrating how analysis of the GALLIUM study data regarding the use of different chemotherapy backbones consistently showed the benefits of obinutuzumab (G) chemotherapy (G-chemo) versus rituximab (R) chemotherapy (R-chemo) in FL patients. An update from the PRIMA study showed that 10-year progression-free survival (PFS) was improved following the use of R maintenance compared with observation following induction. Prof Salles also provided an overview of the RELEVANCE study data, which showed that R plus lenalidomide was not superior to standard R-chemo for the treatment of first-line FL.
Prof Seymour presented data showing that two-thirds of premature FL deaths occur in patients experiencing disease progression within 2 years of treatment, highlighting the need to identify patients at early risk of progression. Prof Seymour explored various prognostic and predictive tools that could be used to identify patients at high risk of death, but he noted that until these prognostic tools are available in the clinic, the high-risk population remains unidentifiable. Furthermore, the accuracy of these prognostic indices needs to be improved. Data analysis from the GALLIUM study showed that G-chemo decreased the risk of a disease progression event in the first 2 years by 46% compared to R-chemo.
Prof Trotman explored the use of PET imaging and detection of minimal residual disease (MRD) to assess treatment outcomes. Prof Trotman showed that PET status at the end of induction is highly prognostic of the outcome. An exploratory analysis of the GALLIUM data showed that the application of the Lugano 2014 response criteria showed a rapid, deep separation of the PFS curves of patients achieving a complete metabolic response (CMR) versus those who did not. There was almost a 5-fold increase in risk of progression and in risk of death in patients failing to achieve CMR. An exploratory analysis of the MRD status of GALLIUM patients showed that a greater proportion of patients in the G-chemo arm achieved MRD-negative status at the end of induction (EOI). Interestingly, patients achieved similar MRD outcomes with G-chemo, regardless of chemotherapy backbone. Both PET and MRD status after induction were independently predictive of PFS.
Dr Pettengell and Mr Bouguet discussed FL from both the clinician’s and the patient’s perspective, with a focus on health-related quality of life (HRQoL). They demonstrated how a marked decrease in HRQoL at progression highlights the importance of extending remission for FL patients. Dr Pettengell presented data from GALLIUM showing comparable quality of life for patients treated with either G-chemo or R-chemo. They also presented patient surveys showing that FL has a substantial physical and psychological impact on patients that both lasts beyond treatment and persists even during long-term remission.
First-Line Treatment of Follicular Lymphoma: Understanding the Key Learnings from the Latest Clinical Data
Professor Gilles Salles
Over a decade ago, studies demonstrated that adding R (the first monoclonal antibody targeting CD20 approved for cancer therapy) to different chemotherapy regimens in FL increased survival compared to chemotherapy alone. A Cochrane Review comparing R-chemo with chemotherapy alone, involving seven randomised controlled studies, demonstrated that R-chemo achieved better overall survival (OS) (hazard ratio [HR] for mortality: 0.63) in comparison to controls.1
Over the past 2 years, a number of studies have increased our knowledge of treating newly diagnosed FL patients: GALLIUM, PRIMA,2 StiL,3 BRIGHT,4 and RELEVANCE5 have all generated important data, which have been presented at key haematology congresses and published in leading journals.
GALLIUM was an open-label, Phase III study in which FL patients were randomised in a 1:1 ratio to receive intravenous G or R in combination with chemotherapy. Choice of chemotherapy (CHOP [cyclophosphamide, hydroxydaunorubicin, oncovin/vincristine, prednisone or prednisolone], CVP [cyclophosphamide, oncovin/vincristine, prednisolone], or bendamustine [benda]) was left to the centre’s discretion. Patients responding at the end of induction received maintenance therapy with the same antibody for 2 years or until disease progression. The data presented showed that, after a median follow-up of 41.1 months, progression-free survival (PFS), assessed by investigators (the primary endpoint of the trial) was 82% for G-chemo versus 75% for R-chemo (HR: 0.68; p=0.0016) (Figure 1).6,7
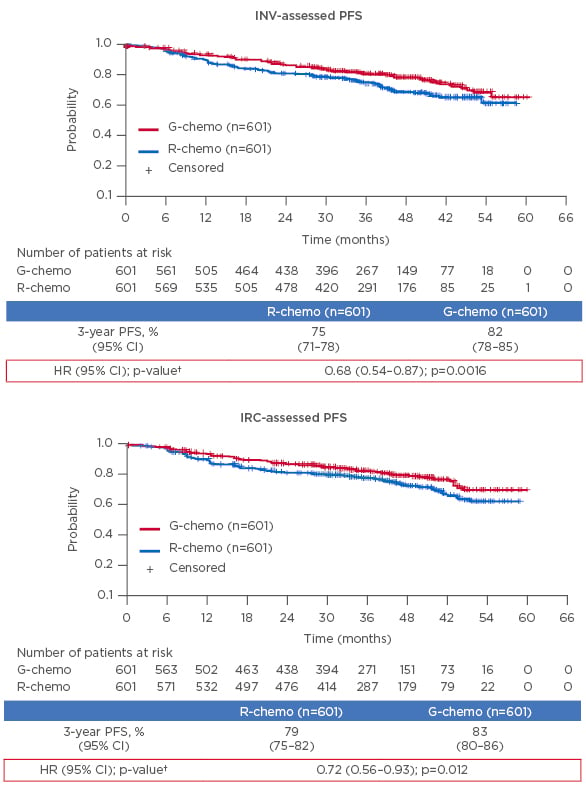
Figure 1: GALLIUM: Progression-free survival after a median follow up of 41.1 months* was significantly improved with obinutuzumab plus chemotherapy versus rituximab plus chemotherapy.
CI: confidence interval; G: obinutuzumab; HR: hazard ratio; INV: investigator assessed; IRC: independent review committee; PFS: progression free survival; R: rituximab.
*Intent-to-treat population in the primary analysis after a median follow-up of 34.5 months, HR for INV-assessed PFS was 0.66 (95% CI: 0.51–0.85; p=0.0012). †Stratification factors: Follicular Lymphoma International Prognostic Index (FLIPI), chemotherapy regimen, and geographic region.
Adapted from Hiddemann et al.6 and Marcus et al.7
Time to next treatment was markedly improved for G-chemo versus R-chemo (HR: 0.68; p=0.007), but there was no significant difference in the OS of the two groups at 3 years. As there are effective second-line therapies for FL, death is a less common outcome than progressive disease. Improved PFS would not necessarily be expected to translate into an OS benefit, especially after only a few years of follow-up in this indolent lymphoma, with estimated median survival >15 years.
Safety data from the chemotherapy backbone analysis showed that Grade 3–5 adverse events occurred in 75% of patients receiving G-chemo versus 69% receiving R-chemo, serious adverse events in 47% receiving G-chemo versus 41% receiving R-chemo, Grade 5 (fatal) adverse events in 4% receiving G-chemo versus 4% receiving R-chemo, and adverse events leading to treatment discontinuation in 16% receiving G-chemo versus 15% receiving R-chemo.6
Analysis of GALLIUM according to different chemotherapy backbones used consistently showed PFS superiority of G-chemo over R-chemo regardless of the chemotherapy paired with it (benda HR: 0.63; CHOP HR: 0.72; and CVP HR: 0.79).6 Analysis of adverse events according to chemotherapy backbone showed that Grade 3–5 adverse events were more frequent for CHOP, while serious adverse events and Grade 5 (fatal) events were more common following benda.6
New data from the PRIMA study, in which patients with high tumour burden and previously untreated FL were randomised in a 1:1 ratio to receive either R-maintenance therapy or observation, was presented at the American Society of Hematology (ASH) 2017 annual meeting.8 Randomisation followed immunotherapy with R plus various chemotherapies. The updated results showed that estimated 10-year PFS was 51% for R maintenance versus 35% for observation (HR: 0.61; p<0.0001).2
In the StiL 1-2003 study, patients with indolent or mantle cell lymphoma (MCL) were randomised in a 1:1 ratio to receive either intravenous benda or CHOP for a maximum of 6 cycles.9 Updated results show that the median time to next treatment had not been reached in the R-benda group versus 56 months in the R-CHOP group (HR: 0.52; p<0.001).10 In both the PRIMA and STiL studies, no OS differences were found between study arms.
The BRIGHT study investigated R-benda versus standard treatment (R-CHOP or R-CVP) in indolent non-Hodgkin lymphoma (iNHL) or MCL.11 After standard treatment was assigned (according to performance status, comorbidities, and general health), patients were randomised in a 1:1 ratio to an open-label treatment with either R-benda or standard treatment. Five-year PFS was 65.5% for R-benda compared with 55.8% for standard treatment (HR: 0.61; p=0.0025).12 When stratified according to lymphoma type, 5-year PFS in iNHL patients treated with R-benda was 81.6% compared with 85% for standard treatment (HR: 0.70; p=0.0582). For MCL, 5-year PFS for R-benda was 39.7% versus 14.2% for standard treatment (HR: 0.40; p=0.0582). However, OS was not statistically different: 81.6% for R-benda patients versus 85.0% for R-CHOP/R-CVP patients (HR: 1.15; p=0.5461). For patients with iNHL, 5-year OS for R-benda was 86.1% versus 89.1% for R-CHOP/R-CVP (HR: 1.34; p=0.3316), while for MCL 5-year OS was 59.4% for R-benda versus 62.6% for R-CHOP/R-CVP (HR: 0.86; p=0.6894).12
Echoing the PRIMA data, in a non-randomised population, a post hoc analysis examining maintenance in BRIGHT (used at investigator’s discretion) found that the duration of response was longer for patients receiving R maintenance after R-benda compared to patients not receiving maintenance (HR: 0.50; p=0.0298).4
The RELEVANCE study was the first randomised Phase III study of a chemotherapy-free regimen, comparing R plus lenalidomide (R2) to standard R-chemo, with investigators given the choice between R-CHOP, R-CVP, or R-benda, followed by R maintenance.13 The RELEVANCE study failed to demonstrate superiority for R2 over R-chemo at 120 weeks for complete response assessed by central review (p=0.13)5 and found no difference for interim PFS (p=0.48).
Future considerations for FL include whether patients at increased risk of early progression can be identified. New therapeutic agents to treat FL include monoclonal antibodies and kinase inhibitors; improve R efficacy through other agents; new targeted agents, such as venetoclax and tazemetostat; and chimeric antigen receptor T cells. Other considerations include integrating new agents into current standards of care and avoiding long-term toxicities.
Recent Developments in Characterising High-Risk Subgroups of Follicular Lymphoma Patients
Professor John Seymour
Although the majority of FL patients treated with chemotherapy, immunotherapy, and maintenance respond well, studies show that approximately 20% experience disease progression within 2 years.9,14-19 Furthermore, a landmark observation showed that two-thirds of premature deaths occur in FL patients experiencing disease progression within 2 years of treatment; this has major implications for providing additional treatment to high-risk patients.19 The study showed an increased Follicular Lymphoma International Prognostic Index (FLIPI)-adjusted risk of death for patients experiencing disease progression within the first 2 years (HR: 6.4; 95% CI: 4.3–9.6); however, patients whose disease remained under control for >2 years had life expectancies similar to age-matched healthy populations. Progression of disease within 2 years (POD24) has been strongly correlated with a higher risk of death and inferior OS.19,20 Two approaches for identifying FL patients at risk of early progression are baseline characteristics prior to treatment and early treatment outcomes, with the former allowing use of tailored treatment or alternative regimens and the later opportunities for adjustment.
FLIPI uses five baseline characteristics (Ann Arbor stage, age, haemoglobin level, number of nodal areas, and serum lactate dehydrogenase) to predict OS and PFS survival. The results from the PRIMA study showed that patients with low FLIPI scores had a 15% risk of early progression compared to 30% for high FLIPI patients.21
FLIPI-2 includes baseline characteristics from FLIPI plus bone marrow infiltration, longest diameter of largest lymph node >6 cm, and β2-microglobulin levels greater than the upper limit of normal.22,23 Studies have shown that FLIPI-2 achieved greater separation between PFS for high, intermediate, and low risk scores, with low score patients having a 10% likelihood of early disease progression.21 FLIPI-2 removes the uncertainty around determining the number of lymph nodes, but requires radiological assessment.
A number of other baseline factors have been identified as potentially prognostic, including blood and bone marrow biomarkers, histological grade, immune phenotype of neoplastic cells, host genetics, microenvironment, and histological transformation.24 The m7-FLIPI score was developed to integrate mutation status of seven genes (EZH2, ARID1A, MEF2B, EP300, FOX01, CREBBP, and CARD11) with FLIPI score and Eastern Cooperative Oncology Group (ECOG) performance status.25 A study showed m7-FLIPI identified a high-risk group (28%) of patients who had 5-year failure-free survival of 38% and a low-risk group of patients (72% of patients) who had a 5-year failure-free survival of 77% (HR: 4.14; p<0.0001). Additionally, the high-risk group had a 5-year OS of 65% versus 90% for the low risk group (HR: 3.38; p<0.001).26 A drawback of m7-FLIPI is the need for next-generation sequencing tools that are not widely available.
In contrast, the PRIMA-prognostic index (PRIMA-PI) used bone marrow involvement and β2 microglobulin as the parameters by which to assess patients, selected because they are easy to measure clinically.21 The final simplified PRIMA-PI score comprises 3 risk categories: low (β2 microglobulin ≤3 mg/L without bone marrow involvement), intermediate (β2 microglobulin ≤3 mg with bone marrow involvement), and high (β2 microglobulin >3 mg/L). Data has shown that PRIMA-PI achieves a similar degree of stratification to FLIPI, but with advantages of simplicity. The 5-year event free survival was 77% for low PRIMA-PI scores versus 76% for low FLIPI scores, 57% for intermediate PRIMA-PI scores versus 64% for intermediate FLIPI scores, and 44% for high PRIMA-PI scores versus 45% for high FLIPI scores.21
An alternative approach is using molecular predictive scores to explore gene expression profiles of 23 genes, selected to reflect multiple aspects of tumour biology.27 The 23 gene expression profile data was found to independently predict progression of high-risk compared to low-risk groups (HR: 3.68; p<0.0001).27 Development of the 23 gene expression panel creates the possibility to integrate information with FLIPI. It has been shown that 80% of patients with both low FLIPI and low 23 gene scores achieve PFS at 10 years. Conversely, patients with high FLIPI and high 23 gene scores are estimated to have a 2-year PFS <50% and should be considered for new treatment options.27
Although the adverse impact of p53 dysfunction has been known for decades in chronic lymphocytic leukaemia, the risk of TP53 gene mutation and p53 protein expression has only recently become apparent in FL. Analysis of somatic mutations in 94 genes from 277 FL patients revealed 10 genes (including TP53) were more frequently mutated in early progression. Moreover, data showed that TP53 expression occurred in 18% of patients with early progression versus 2% with late progression.28 In a second study evaluating p53 expression in PRIMA, investigators found p53 expression in >20% of cells was associated with reduced PFS (HR: 1.53; p=0.048), but not significantly associated with worse OS.29 Such data suggests p53 expression represents an important prognostic factor.
A study exploring GALLIUM data showed that OS at 2 years was 82.4% for FL patients who had progressed in the first 24 months (POD24) versus 98.2% for patients with no POD24 (age-adjusted HR: 12.2).20 The presentation demonstrated that the use of G-chemo versus R-chemo was associated with a 46% reduction of POD24 over 2 years (Figure 2).
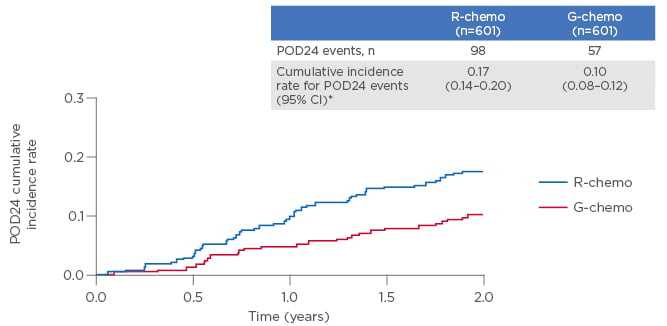
Figure 2: Exploratory analysis from the GALLIUM study: In first-line follicular lymphoma, obinutuzumab plus chemotherapy reduced the risk of a POD24 event over 2 years by 46% versus rituximab plus chemotherapy.20
CI: confidence interval; G: obinutuzumab; POD24: progression of disease within 2 years; R: rituximab.
*2-year cumulative incidence rates.
Risk reduction based on (1-HR)x100. Following further analysis, data value has been revised to 46% (95% confidence interval: 25.0–61.1) (as of November 2017).
The POD24-Prognostic Index (POD24-PI), consisting of four parameters (high-risk FLIPI, EP300, FOX01, and EZH2), was designed to predict POD24. Comparison of the abilities to predict POD24 from the German Low-Grade Lymphoma Study Group (GLSG) data showed that m7-FLIPI was 76% accurate, POD24-PI 71% accurate, and FLIPI 60% accurate.26
Analysis of POD24-PI in GALLIUM demonstrated that the earlier progression occurs, the greater the risk of mortality. In the study, OS at 2 years was 20% for patients who experienced POD at 6 months, 58.4% for patients who experienced POD at 12 months, and 76.5% for patients who experienced POD at 18 months.20
Other potential prognostic parameters include total metabolic tumour volume (TMTV), circulating tumour cells (CTC), and cell-free DNA (cfDNA). Significant correlations have been found between TMTV and both CTC and cfDNA. A study showed that 4-year PFS was lower in FL patients with TMV >510 cm3, CTC >0.0018 peripheral blood cells, and cfDNA >2,550 equivalent-genome/mL.30 In comparison with TMTV alone, no additional prognostic information was obtained measuring CTCS, but PFS was shorter for patients with high cfDNA and TMTV suggesting each independently identified outcomes.
To conclude, optimal implementation of FL prognostic tools has yet to be achieved and the high-risk population of FL patients remains unidentifiable.
Promising New Ways to Assess the Outcome of Follicular Lymphoma Treatment: Applying GALLIUM Data in the Real World
Professor Judith Trotman
Prof Trotman presented two promising new approaches for the assessment of FL outcomes. For patients in the GALLIUM trial, EOI PET scan information was available for 595 patients, MRD status in 785 patients, and both in 298 patients.6,31,32
Analysis of EOI response (according to 2014 Lugano criteria)33 revealed no significant differences in outcomes between G-chemo and R-chemo (OR 84% for G-chemo versus 79% for R-chemo [p=0.30]).32 Furthermore, complete metabolic response (CMR) was 78% for G-chemo versus 73% for R-chemo (p=0.18). One possible explanation for a lack of difference in CMR rate may be due to the study not being sufficiently powered (n=595) to address metabolic response.32-34 There was a rapid, pronounced, and ongoing separation in PFS between patients achieving CMR and those failing to do so (HR: 0.21; 95% CI: 0.13–0.34; p<0.0001).32 Additionally, the Lugano 2014 criteria showed a significant difference in OS between patients achieving CMR at EOI and those failing to do so (HR: 0.22; 95% CI 0.11–0.45; p<0.0001).32 Within 2.5 years after induction, 13% (9/69) of patients failing to obtain CMR had died from their lymphoma compared to 1% (5/450) of those achieving CMR.
In a Cox multiple regression model, the only highly significant predictor of inferior OS was failure to achieve CMR (HR: 0.2, p<0.0001). PET status (non-CMR versus CMR), treatment arm (R-chemo versus G-chemo), induction chemotherapy (benda versus CHOP/CVP), and FLIPI category did not prove to be strong predictors of inferior OS.31
Analysis of GALLIUM demonstrated minimal residual disease (MRD) response (using blood and bone marrow) was 92% with G-chemo versus 84.9% with R-chemo (p=0.0041).31 For bone marrow, MRD was 93% with G-chemo versus 82.5% with R-chemo arm (p=0.0014).31
Additionally, analysis of GALLIUM showed MRD response rates were 92% for G-benda, 91% for G-CHOP, and 91% for G-CVP, suggesting obinutuzumab may act as an equaliser for less efficacious chemotherapies (Figure 3).31
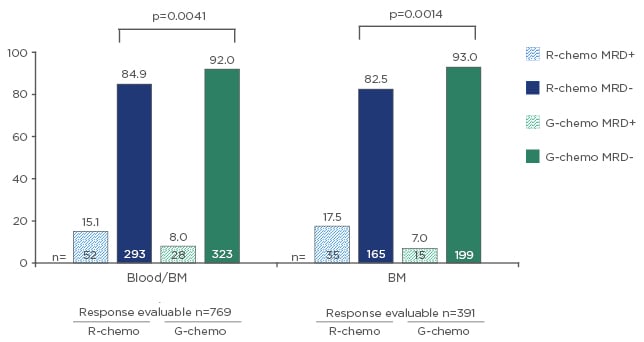
Figure 3: Results from the GALLIUM study: Higher minimal residual disease response with obinutuzumab plus chemotherapy versus rituximab plus chemotherapy across compartments at the end of induction (N=785).
BM: bone marrow; G: obinutuzumab; MRD: minimal residual disease; R: rituximab.
Adapted from Pott et al.31
In the patient group with both CMR and MRD-negative response, 2.5-year PFS (from EOI) was 85% (95% CI: 80–89), compared with 69% in the CMR and MRD-positive population.
During front-line management, awareness needs to be raised that agents increasing depth and duration of remission can cause immune suppression leading to an increased risk of infection. Such observations raise questions around the trade-offs that are made by patients at different times in their FL management.
Maintaining Quality of Life in the Treatment of First-Line Follicular Lymphoma: Patient Perspective
Doctor Ruth Pettengell and Mr Guy Bouguet
Dr Pettengell and Mr Bouguet provided an overview of the patient journey from diagnosis to remission from both clinical and patient perspectives. Information regarding the patients’ perspective of the treatment journey was taken from the 2018 Lymphoma Coalition Global Patient survey, with 6,600 respondents, including 937 patients with FL.35
At diagnosis, clinicians focus on staging and risk assessments to gain insights into disease course, with clinical activities involving biopsy and grading, CT/PET, and blood count and chemistry. Prognostic scores are used to determine treatment options, whether radiotherapeutic, ‘watch and wait’, or immunochemotherapeutic approaches are used. For treatment management, the most important aspects are minimising the impact of FL, treatment-related side effects, and quality of life.36
From the patient perspective, tailored information at diagnosis about FL and treatments is important. Patients want access to treatment and facilities, drug reimbursement, and personal support, and place emphasis on their ability to perform daily activities, as well as their physical, emotional, and psychological wellbeing.35,37,38
Quality of life depends on which stage of the treatment journey the patient has reached. At diagnosis, disease-related symptoms (e.g., enlarged lymph nodes and drenching night sweats) have the biggest impact, but, during treatment, emphasis shifts towards side effects (e.g., nausea/vomiting).39
Given that FL currently cannot be cured, treatment aims include minimising the number of organs affected, controlling symptoms, improving quality of life, and promoting prolonged remission.37,40 Patients want the most effective option first to achieve longer treatment-free periods and avoid repeated chemotherapy. The lymphoma survey showed patients most actively sought information at diagnosis (64%), followed by 1–3 months later (19%).35 The survey found 65% of respondents would have liked more information and support at diagnosis, compared to 22% who felt they had received sufficient levels of support. Doctors and websites were a source of information for 65% of patients, while 43% of patients also reported using patient organisations as a source of information. Only 33% of patients felt this information to be adequate, raising concerns around lack of information, which gives rise to confusion, stress, and fear.35
Clinical considerations around treatment initiation include tumour burden, grade, symptoms, and comorbidities.41 For patients, one of the main considerations is efficacy versus toxicity, with patients also wanting good quality of life, prognosis, drug reimbursement, and communication with health professionals. Their stage in life (e.g., whether in education, working, or retired) can influence their perspective.
The ‘watch and wait’ concept may confuse patients, with many concerned they are not receiving treatment. One study showed patients undergoing active FL surveillance experienced comparable scores for fatigue, sleeping problems, and financial problems to patients receiving radiotherapy and chemotherapy.42 Such findings demonstrate the fear that results from poor communication.
Options for first-line treatment of symptomatic FL are R monotherapy or CD20 monoclonal antibodies with chemotherapy (most commonly CHOP, benda, or CVP), followed by CD20 monoclonal antibody maintenance.7,36,43,44 Patient needs depend on factors including age, previous treatment, disease stage, and life situations. Younger patients with a high-risk of progression can be willing to accept greater impact from treatment in return for efficacy, while older patients may look to balance treatment efficacy with quality of life. Initially, patients commonly feel efficacy to be paramount, but may prioritise quality of life after experiencing adverse events. Such shifting views demonstrate the importance of communication to align physician and patient goals.
Due to increased longevity, HRQoL has become an increasingly important consideration. The NCCN Functional Assessment of Cancer Therapy lymphoma (FACT-Lym) questionnaire has 42 items validated in lymphoma patient populations.45 A study administering FACT-Lym to FL patients showed scores differed according to disease stage, with relapsed patients demonstrating the lowest scores.46
A community-based study from the USA exploring the physical symptoms, side effects, and impaired performance in FL patients showed that, after first-line therapy, HRQoL of patients receiving R was equal to that of those undergoing observation.47 Exploring HRQoL in GALLIUM, comparable improvements were found for G-chemo and R-chemo during maintenance and follow-up.48
During remission, there is debate over whether patients should be offered intermittent appointments or make appointments when they experience concerns. Data from the patient survey shows aspects of wellbeing (such as fear of relapse and loss and inability to work) get worse during remission.35 Although the impact of FL on wellbeing improves with time, fear of relapse persists for >8 years after the end of treatment.35
In conclusion, maintaining remission in patients optimises HRQoL, with the GALLIUM study showing HRQoL improves during and after induction that remain stable during maintenance and remission. The persistent fear of relapse cited by patients suggests the need for continuous support during the treatment journey. Overall, patient organisations, physicians, and allied healthcare professionals need to support patients throughout their journey.







