Meeting Summary
The main objectives of the symposium were to explore the current developments in the diagnosis and treatment of non-Hodgkin lymphoma (NHL). An overview of the hurdles and unmet needs in the management of indolent NHL were discussed, followed by the current and future perspectives for the treatment of indolent NHL. The topic of frontline treatment outcomes in diffuse large B-cell lymphoma (DLBCL), the most common type of high-grade NHL, was also explored with an emphasis on how outcomes could be improved.
Hurdles and Unmet Needs in the Management of Indolent Non-Hodgkin Lymphoma
Professor Gilles Salles
Prof Salles reviewed the current clinical management of indolent NHL, including epidemiological data, disease biology, heterogeneity, and progression. Learnings from these findings provided insights into current treatment goals.
The incidence of follicular lymphoma (FL) has almost doubled from the 1970s to the 21st century.1 A survey conducted by the large collaborative group, InterLymph, revealed a number of FL risk factors.1 An increase in FL risk was reported for individuals who had a first-degree relative with NHL (odds ratio [OR]: 1.99); had a greater BMI as a young adult (OR: 1.15); worked as spray painters (OR: 2.66); or had Sjögren’s syndrome (OR: 3.37). Conversely, FL risk was reduced in individuals who had asthma, hay fever, or food allergy (OR: 0.79–0.85); had undergone blood transfusions (OR: 0.78); or had high recreational sun exposure (OR: 0.74).1 At the genetic level, a strong association was found between high levels of t(14;18) translocation in prediagnostic blood samples from apparently healthy individuals and development of FL; while there was no absolute threshold for the level of t(14;18) translocation, individuals with t(14;18) frequency reaching one in every 10,000 blood cells had a 23-fold greater risk of progression to FL.2
In FL, cells carry abnormalities which develop over time and lead to worse outcomes for overall survival (OS) with age.3 In patients that relapse, analyses of clonal populations previously present in tumour nodes or serum were found to have diverged over time, emphasising the complexity of clonal evolution (Table 1).4,5

Table 1: Hurdles and unmet needs in follicular lymphoma.
Gene expression analyses have highlighted the prognostic role of the tumour microenvironment, drawing correlations between increased macrophage count with worse outcomes;6 these results were corroborated in a second independent study,7 and partly reproduced by some8,9 but not other studies.10-12 Discrepancies in the latter findings may be attributed to changes within the microenvironment governed by patient population and treatment regimen. In patients with FL treated with rituximab plus combination chemotherapy of cyclophosphamide, adriamycin, vincristine, and prednisone (R-CHOP), the association between macrophage content and prognosis was reversed.13
In an effort to improve clinical indexes by mutational analysis or gene expression profiling, a team of German and Canadian investigators grouped seven mutated genes (EZH2, ARID1A, MEF2B, EP300, FOXO1, CREBBP, and CARD11) and analysed them together with clinical factors in a model termed m7-FLIPI and refined this index by monitoring progression of disease (POD) at 24 months.14 Based on mutation clusters, patients were classed into three risk categories: high, intermediate, and low. The m7-FLIPI model was correlated with survival probability and showed that patients in the high-risk group had worse 5-year failure-free survival versus low-risk patients (38.29% versus 77.21%; hazard ratio [HR]: 4.14; 95% confidence interval [CI]: 2.47–6.93; p<0.0001).14 OS was also significantly worse in patients classified as high-risk by the model compared with low-risk patients. These results were echoed in a recent study showing that, when assessed at the 5-year time point, patients who failed primary therapy within 2 years after diagnosis (study cohort) had a 50% probability of survival compared with about 90% in the reference group.15 Furthermore, re-treatment in these patients resulted in lower response rates and shorter response durations.16
In the PRIMA study, biopsies were assessed for histologic transformation (HT), an adverse event (AE) in the natural history of lymphoma, at first POD in FL patients who previously responded to immunochemotherapy. Almost 80% of patient biopsies had FL histology, of which 20% had HT. POD with HT appeared to occur early, with a median relapse time of 10 versus 23 months in populations with and without HT, respectively.17 Clonal variation and clonal prevalence were examined from cellular biopsies of patients during relapse or during transformation. In patients without a transformation, clonal populations, although varied over time, were made up of the same few clones over the evolution of lymphoma. In patients with a HT, however, new clones emerged over time that were not detected at diagnosis.18 Earlier relapse may increase the likelihood of transformation, but at present we lack the molecular tools at least using mutational analysis to predict transformation. Current treatments provide some benefit to patients, but are also linked with a range of side effects associated with chemotherapy, including fatigue, alopecia, development of neuropathy, and heart failure. Patients may also be at an increased risk of developing secondary AML, myelodysplastic syndrome, or potentially, secondary tumours.19,20
Current treatments enable us to treat most tumours, but it becomes increasingly difficult when patients experience HT. Therefore, finding an ideal therapeutic target for the treatment of FL remains to be identified. To address this issue, newer therapies may need to target self-renewing cancer progenitor cells to halt FL progression and transformation. Of interest is that the presence of the lymphoma-associated t(14;18) translocation in B cells can appear many years before disease manifestation in FL and confers a survival advantage for the mutated B cell by preventing apoptotic death. However, this translocation alone appears to be insufficient to drive development of FL and must be followed by a secondary oncogenic event. How these cells traffic through germinal centres and receive antigenic triggers to evolve to FL precursor cells is a question of major interest. There may be secondary events leading to the generation of cancer progenitor cells, which may proliferate and give rise to tumours. We may have the tools to treat tumours, but this becomes increasingly difficult after tumour transformation. What these data show is that there is a need to target these precursor cells to eliminate tumours in the hope of curing patients.
Progress and Promise: Current and Future Perspectives on Therapy of Indolent Non-Hodgkin Lymphoma
Professor Nathan H. Fowler
Clinical trials in FL have progressed over the last 30 years, from treatment regimens containing bleomycin, interferon, and very intensive regimens of alternating triple therapy, to treatments such as rituximab plus fludarabine, mitoxantrone, and dexamethasone. Although OS rates improved with advances in treatment regimens, improvements in failure-free survival were modest, and most patients still relapsed in the first several years following induction therapy.21
One of the first randomised studies to investigate the most effective chemotherapy regimen to combine with rituximab for first-line therapy of advanced FL was the FOLL-05 trial in 2013.22 The study highlighted that rituximab was commonly used for patients with advanced FL, but there was no clear optimal associated chemotherapy regimen. In this landmark study, 500 treatment-naïve patients with ‘active’ FL were randomised (1:1:1) to rituximab plus cyclophosphamide, vincristine, prednisone (R-CVP); R-CHOP, or fludarabine, mitoxantrone (FM).22 R-CHOP and R-FM were found to be superior to R-CVP in progression-free survival (PFS), but not OS, which was similar across treatment arms.22,23 However, the FM group had higher rates of infection and cytopenia, and increased risk of secondary cancers.22 R-CHOP offered the longest disease control, with better overall outcomes compared with R-FM or R-CVP. In the same year, the StiL NHL1 study reported R-CHOP treatment versus the bendamustine, rituximab (BR) regimen in patients with newly diagnosed Stage III or IV indolent or mantle-cell lymphoma, with the primary objective of assessing non-inferiority of BR versus R-CHOP for PFS.24 BR demonstrated superiority over R-CHOP for PFS (31.2 versus 69.5 months; p<0.00001), but not for OS over a 5-year (Figure 1) or 10-year period.24,25
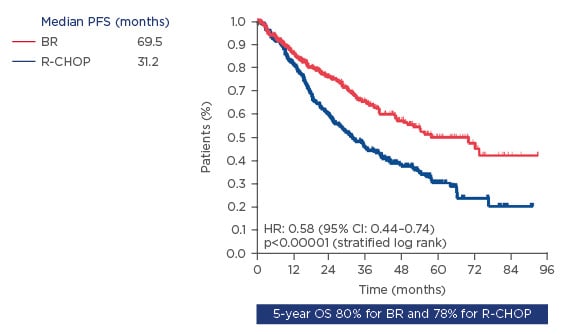
Figure 1: Progression-free survival in patients with indolent and mantle-cell lymphomas on BR versus R-CHOP as first-line treatment.
BR: bendamustine, rituximab; CI: confidence interval; HR: hazard ratio; OS: overall survival; PFS: progression-free survival; R-CHOP: rituximab plus cyclophosphamide, adriamycin, vincristine, and prednisone.
In the 2014 BRIGHT study, BR was found to be non-inferior to R-CVP or R-CHOP for complete response rate when assessed in treatment-naïve patients with indolent NHL or mantle cell lymphoma.26 Both regimens were effective, but no difference was observed in PFS and OS. Therefore, treatment selection should be based on toxicity associated with treatment, as well as schedule and patient selection. In 2011, the PRIMA study was initiated to assess the potential benefit of 2 years of rituximab maintenance after first-line treatment in patients with high tumour burden FL. The study was designed to show a 45% improvement in median PFS after randomisation when rituximab was given as maintenance in patients who had received R-CHOP, R-CVP, or R-FCM (fludarabine, cyclophosphamide, mitoxantrone) as first-line therapy. The study demonstrated the sustained and persistent benefit rituximab maintenance after immunochemotherapy with a 6-year PFS estimate of 59.2% in the rituximab maintenance arm versus 42.7% in the observation arm.27,28
Obinutuzumab, a novel Type 2 humanised anti-CD20 monoclonal antibody engineered for improved antibody-dependent cell-mediated cytotoxicity, was tested for efficacy against rituximab in the 2016 GALLIUM study.20 Patients with previously untreated, CD20-positive FL, or splenic/nodal/ extranodal marginal zone lymphoma were assigned to receive standard chemotherapy with CHOP, CVP, or bendamustine and then randomised (1:1) to rituximab or obinutuzumab.20 Significantly better outcomes for PFS were reported in the obinutuzumab-based regimen (p<0.001).20
Obinutuzumab-based chemotherapy was also assessed in patients receiving bendamustine who were refractory to rituximab (failed rituximab within 6 months of chemotherapy) in the 2016 GADOLIN study.29 The addition of obinutuzumab significantly improved PFS in these patients (HR: 0.55; 95% CI: 0.40–0.74) and also in patients who were refractory to both alkylators and rituximab (HR: 0.56; 95% CI: 0.40–0.78). Positive data from this trial led to US Food and Drug Administration (FDA) approval of obinutuzumab with bendamustine for the treatment of FL in the relapsed setting. Although these studies are evidence of our incremental progress in FL, most patients are still at risk of relapse in 5–10 years and individual patient responses to therapy still vary greatly. Recent trials have provided valuable insights into the influence of the immune microenvironment in prognosis of patients with FL. OS has been correlated with the molecular features of non-malignant immune cells present in the tumour at diagnosis, underscoring the significance of the tumour microenvironment in clinical outcomes.6 Such analyses have led to the identification of several key cellular pathways in FL, as well as other lymphomas which are now targetable, including B-cell receptor signalling.30
There has been some progress in treating relapsed/ refractory patients, who were failing rituximab and were alkylator-refractory, with phosphatidylinositol-3-kinase inhibitors such as idelalisib,31 duvelisib,32 and copanlisib.33 These agents are associated with unique toxicity profiles; idelalisib is associated with nausea and liver function test abnormalities in the first or second cycle. There is also diarrhoea, colitis, and infectious complications. Therefore, it is important to carefully consider the benefits of these agents against their potential side effects in this difficult-to-treat population. Positive data have also been observed with the Bruton’s tyrosine kinase (BTK) inhibitor, ibrutinib, hypothesised to improve patient response to rituximab due to its ability to modify the tumour microenvironment. Indeed, high response rates have been observed (82%) when ibrutinib is used in combination with rituximab. AE were primarily Grade 1–2 and in line with the safety profile for ibrutinib.34 Lenalidomide has also been shown to have excellent synergy with rituximab in patients with untreated, advanced stage indolent NHL.35 Five-year PFS was reported as 65% (48% for marginal zone lymphoma and 50% in small lymphocytic lymphoma)34 with similar findings reported in the ALLIANCE study in previously untreated FL patients.36 In these and other studies in relapsed/refractory FL, the most common Grade 3–4 AE were neutropenia and thrombocytopenia. Lenalidomide plus rituximab is also being investigated for efficacy versus chemotherapy plus rituximab in treatment-naïve patients with histologically confirmed FL Grade 1, 2, or 3a, Stage II–IV in the RELEVANCE trial;37 versus placebo plus rituximab in patients with relapsed/ refractory indolent NHL in the AUGMENT trial,38 and as maintenance in relapsed/refractory NHL in the MAGNIFY trial.39
With most current regimens offering similar PFS, treatment should be assessed on an individual basis for patients, bearing in mind the treatment schedule and associated toxicities. Furthermore, considering advances in our understanding of FL biology and development of novel agents with potentially better outcomes, it may be time to look to next-generation agents in an effort to change the natural history of the disease.
Improving Frontline Treatment Outcomes in Diffuse Large B-cell Lymphoma
Professor Umberto Vitolo
Treatment options available in clinical practice for patients with aggressive lymphoma, specifically for DLBCL, include standard R-CHOP therapy. Sixty percent of patients who have DLBCL respond well to R-CHOP and can be cured of disease,40 but for the remaining 40% of patients who fail R-CHOP, the chances of salvage are poor.41 Patients with early relapse and those refractory to prior rituximab treatment have poor response to salvage chemotherapy. Poor outcomes are also observed in young patients undergoing high-dose chemotherapy (despite receiving autologous stem cell transplantation) and in elderly patients with existing comorbidities. Thus, the main goal for DLBCL patients is to improve the efficacy of standard first-line treatment, i.e. R-CHOP. Alternative strategies for these patients may involve: substitution of rituximab with obinutuzumab; intensification of chemotherapy (DA-EPOCH-R); and additional maintenance or inclusion of a novel agent in the existing R-CHOP regimen (X-R-CHOP).
In the GOYA study, 1,418 patients were randomised (1:1) to receive R-CHOP as standard regimen or G-CHOP (CHOP plus obinutuzumab) for six to eight courses with PFS as the primary outcome; however, no significant difference was observed between the treatment arms with regard to PFS or OS.42 Another approach taken to improve the outcome of DLBCL was to intensify chemotherapy. This was assessed in the Cancer and Leukemia Group B (CALGB)/Alliance Phase III, randomised R-CHOP versus DA-EPOCH-R study in patients with Stage II–IV newly diagnosed DLBCL with event-free survival as the primary endpoint.43 Again, no significant difference between treatment arms was observed, but there was a significant increase in haematologic toxicity in the dose-adjusted EPOCH-rituximab arm.43 In the REMARC study, where elderly patients who responded to R-CHOP were randomised to receive either the immunomodulatory agent, lenalidomide, or placebo as maintenance therapy, an improvement in the primary endpoint, PFS, was reported in the lenalidomide maintenance arm versus placebo (HR: 0.708; 95% CI: 0.537–0.932), but this did not translate into an OS benefit (HR: 1.218; 95% CI: 0.861–1.721) between treatment arms (Figure 2).44
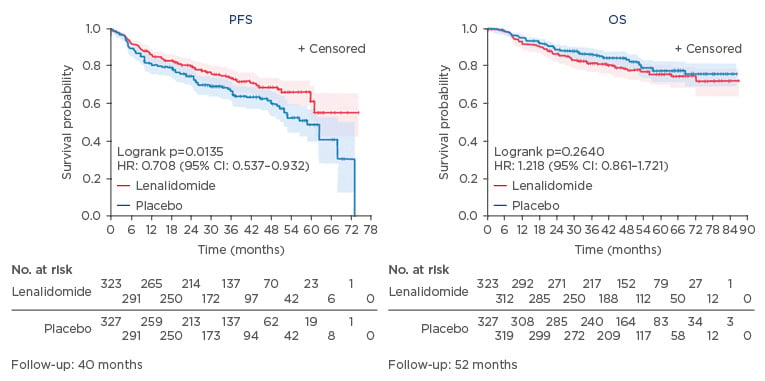
Figure 2: Lenalidomide maintenance after R-CHOP in elderly DLBCL patients.
CI: confidence interval; DLBCL: diffuse large B-cell lymphoma; HR: hazard ratio; OS: overall survival; PFS: progression-free survival; R-CHOP: cyclophosphamide, adriamycin, vincristine, and prednisone.
Addition of novel agents to standard R-CHOP is the newest strategy under investigation for improving outcomes in the treatment of DLBCL. In a small, non-randomised Phase Ib study, ibrutinib was administered in combination with R-CHOP in patients who had untreated CD20-positive B-cell NHL. Almost all patients responded to treatment, and 70% of them achieved complete remission; however, this was at the expense of a 70% increase in Grade 4 neutropenia.45 Lenalidomide plus R-CHOP (R2-CHOP) is also currently under investigation, based on its established immunomodulatory profile and its activity in combination with rituximab.46 Promising results have been reported from two Phase II trials of R2-CHOP in frontline DLBCL, with objective response rates of 98% and 92% and complete response of 80% and 86% reported for the respective studies.47,48 As evidenced from other studies where additional treatments were added to R-CHOP, there is an observed increase in AE, in particular neutropenia. However, this did not translate into an excess rate of infection or febrile neutropenia, maintaining a similar safety profile to that expected from R-CHOP alone.47,48
In addition to exploring new treatment options or combinations, potential strategies to improve patient outcomes include proper identification of poor-prognosis subsets of DLBCL. This includes patients with double-hit lymphoma, who were found to have poor outcomes regardless of induction regimen.49 Early identification of high-risk patient populations, as defined by concurrent expression of MYC and BCL2 proteins or concurrent presence of MYC and BCL2 translocations, can be critical in guiding therapeutic decisions.50,51
Tailoring treatment selection based on genetic or phenotypic characteristics is another option to improve outcomes. Gene expression profiling (GEP) studies in DLBCL have demonstrated that histologically uniform subtypes are biologically distinctive on the molecular level on the basis of their cell of origin (COO). However, because standard GEP tests initially required fresh-frozen biopsy tissues, their use in daily clinical practice was limited. Surrogate immunohistochemistry-based biomarker tests were developed, which, while less sensitive than GEP, could be adopted in a wide range of clinical haematopathology diagnostic laboratories.52,53 The recent development of a GEP assay for COO classification using formalin-fixed paraffin-embedded tissue sections and a simplified digital gene-expression platform (Lymph2Cx, NanoString Technologies Inc., Seattle, Washington, USA) that can be more easily adopted into clinical practice, is currently under evaluation in a number of clinical trials.
Genetic analyses have revealed two major subtypes: germinal centre B-cell-like (GCB) and activated B-cell-like (ABC), and an additional small subgroup could not be precisely classified into these two entities. Patients with the ABC subtype have been shown to have a lower chance of cure with standard therapy, thus the ability to personalise treatment choices based on molecular subtype can identify patients with a high unmet medical need for non-standard therapy.
When tested in patients treated with R-CHOP, those with the GCB subtype did significantly better than those with ABC.54 Further studies in these patient subgroups revealed more selective activity of lenalidomide and ibrutinib in the ABC population.55,56 Ibrutinib was shown to have preferential clinical activity in the ABC subtype in an interim analysis of a Phase II study; ibrutinib produced complete or partial responses in 37% (14/38) of those with ABC DLBCL, but in only 5% (1/20) of subjects with GCB DLBCL (p=0.0106).56 A hallmark of the ABC subtype is constitutive activation of the NF-κB pathway and as BTK is required for NF-κB signalling in ABC, BTK inhibitors work well in these patients. Lenalidomide was shown to significantly improve PFS over investigator’s choice (gemcitabine, rituximab, etoposide, or oxaliplatin) (p<0.05), with greater improvements in non-GCB (15.1 versus 7.1 weeks, respectively; p=0.02) compared with GCB (10.1 versus 9.0 weeks, respectively; p=0.55) patients.57 Similar benefits of lenalidomide on event-free survival were reported when it was added to R-CHOP in ABC patients,47,58 likely via lenalidomide’s immunomodulatory effect on T cell and natural killer cell function in the tumour microenvironment. While R-CHOP remains the standard of care in DLBCL and the backbone of new treatments with novel compounds, ongoing studies such as PHOENIX and ROBUST will provide further insight into the efficacy and safety of ibrutinib and lenalidomide in combination with R-CHOP in the harder-to-treat ABC patient population.59,60







