Meeting Summary
Patients with refractory/relapsed (R/R) non-Hodgkin lymphoma (NHL) make up a very heterogeneous population with a poor life expectancy. The objective of this symposium was to provide an overview of the current treatment landscape for aggressive NHL, as well as the future research on new treatments. Transplant-eligible patients receive salvage chemotherapy, followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT). Patients who fail transplant or are transplant-ineligible generally receive palliative treatment or enter clinical trials; there is no standard of care and thus there is a high unmet clinical need. Pixantrone is currently indicated for adult patients with multiply R/R aggressive B-cell NHL, thereby filling the unmet clinical need in this field. The symposium started with a brief overview of the meeting objectives. This was followed by an overview of the current and future treatment landscape for aggressive NHL, including a case study of a patient with diffuse large B-cell lymphoma (DLBCL) with multiple relapses receiving pixantrone as monotherapy. The results and post hoc analysis of the CORAL and the SCHOLAR1 studies were reviewed, including the relative merits of combination therapy versus monotherapy for patients with relapsed DLBCL who had failed second-line salvage therapy. The symposium ended with an outline of the profile and mechanism of action of pixantrone, and evidence from the PIX301 study that provided the basis for regulatory approval for the use of pixantrone in third and fourth-line treatment of R/R aggressive B-cell NHL. The clinical efficacy and safety of pixantrone were reviewed, together with a future perspective on the ongoing PIX306 trial. The symposium concluded with the presentation of two clinical cases of patients treated with pixantrone, a ‘Question and Answer’ session, and a panel discussion.
Treatment Landscape of B-Cell Non-Hodgkin Lymphoma Patients, Looking into the Future of New Strategies
Professor Pier Luigi Zinzani
Treatment Landscape of Aggressive B-Cell Non-Hodgkin Lymphoma Patients
The majority (about 85%) of NHL arise from B-lymphocytes, with DLBCL being the most common subtype (37%).1 Although aggressive B-cell NHL has a cure rate of approximately 60%, relapse within the first 2 years following initial therapy of rituximab with cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine, and prednisolone (R-CHOP) is common.2
There is currently no recognised standard of care for patients who fail first and second-line treatment and who are not eligible for SCT. Market research conducted in 2016 among a group of 154 haemato-oncologists and oncologists in France, Italy, and Spain demonstrated that ≥9 different regimens (including monotherapy and combination therapy) may be used in third and fourth-line settings.3 The life expectancy of the multiple relapsed population is poor;2 as such, there is a significant unmet medical need in patients with multiply R/R-DLBCL as well as those ineligible for SCT.
Prognostic Factors and New Treatment Options in Diffuse Large B-Cell Lymphoma
Gene-expression profile studies have revealed three molecular subtypes of DLBCL according to the cell-of-origin: germinal-centre B-cell-like DLBCL, activated B-cell-like (ABC) DLBCL, and primary mediastinal B-cell lymphoma.4,5
A number of novel single-agent therapies for relapsed DLBCL ineligible for SCT are currently in development. These include:
- Bruton’s tyrosine kinase (BTK) inhibitor (ibrutinib)
- Phosphoinositide 3-kinase (PI3K) inhibitors (including buparlisib [BKM120], copanlisib [BAY80-6946], and TGR-1202)
- BCL2 inhibitor (venetoclax)6
- Antibody drug conjugates (denintuzumab)
- Small molecule inhibitor of exportin 1 (XPO1) inhibitor (selinexor)7
- Checkpoint inhibitors (pembrolizumab and nivolumab)8
- EZH2 inhibitor (tazemetostat)9
- Chimeric antigen receptor T-cell (CAR-T) therapy10
Overall, none of the drugs currently being evaluated show potential as an active single agent in the treatment of patients with relapsed DLBCL ineligible for SCT. However, preliminary data with CAR-T are promising; complete response (CR) rates of 50–60% have been observed in R/R-DLBCL,11 although a number of safety issues have been noted, including cytokine release syndrome and B-cell aplasia.10
With regard to combination therapies, a Phase Ib/II programme evaluating combination therapies of ibrutinib, lenalidomide, and rituximab in patients with R/R non-germinal-centre B-cell-like subtype DLBCL is currently ongoing.12
Currently, pixantrone is the only single-agent treatment approved by the European Medicines Agency (EMA) for the management of aggressive B-cell NHL progressing after two or more prior lines of therapy.13 The benefit of pixantrone treatment has not been established in patients when used as fifth-line or greater chemotherapy in patients who are refractory to last therapy. Pixantrone was approved by the EMA primarily on the basis of the results from the PIX301 study, which evaluated the efficacy and safety of pixantrone as a singleagent therapy in the management of patients with aggressive R/R-NHL who had received at least two prior therapies.13,14 In a post hoc analysis of data from the PIX301 study, pixantrone was shown to be more effective than active comparator in the above-mentioned setting, independent of previous rituximab therapy.15 In a historical comparison, pixantrone as a single agent in patients with two or more prior chemotherapy lines had a similar CR rate (about 27%) with salvage regimens, such as rituximab plus etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin (hydroxydaunorubicin) (R-EPOCH) or rituximab plus ifosfamide, carboplatin, and etoposide (R-ICE), although it should be noted that the studies are not directly comparable.14,16-19
Case Study
In January 2010, a 36-year-old male presented with bulky axillary lymphadenopathy and was diagnosed with DLBCL with an ABC-like phenotype.20 Computerised tomography and positron emission tomography scans demonstrated multiple lymphadenopathies above and below the diaphragm and skeletal involvement confirming Stage IV disease. The treatment plan is depicted in Figure 1. Following two relapses, pixantrone was administered for six 28-day cycles; CR was observed at Cycle 3. At the end of treatment, CR was confirmed by positron emission tomography and is ongoing after 17 months.
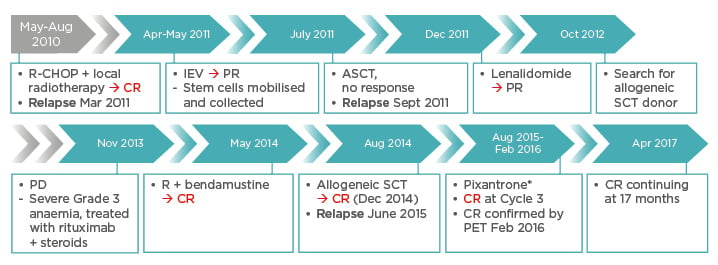
Figure 1: Case study treatment regimen.20
*50 mg/m2 on Days 1, 8, and 15, for six 28-day cycles; 20% dose reduction due to risk of
haematological toxicity.
ASCT: autologous stem cell transplantation; CR: complete response; IEV: ifosfamide plus etoposide and epirubicin: PET: positron emission tomography; PD: progressive disease; PR: partial response; R: rituximab; R-CHOP: rituximab plus cyclophosphamide, doxorubicin (hydroxydaunomycin), vincristine, and prednisolone; SCT: stem cell transplantation.
In summary, many new therapies are in development for the treatment of R/R aggressive NHL in patients who failed or are not eligible for SCT. However, at present, pixantrone is the only approved treatment in multiply-relapsed aggressive NHL.
Patients with Relapsed Diffuse Large B-Cell Lymphoma Who Fail Second-Line Salvage Therapy: Monotherapy Versus Combination Therapy
Professor Eric Van Den Neste
CORAL and SCHOLAR1 Studies
The CORAL study evaluated rituximab plus dexamethasone, cytarabine (high-dose Ara-C), and cisplatin (R-DHAP) versus R-ICE in patients with DLBCL.18,21 Patients who were chemosensitive received carmustine, etoposide, cytarabine, and melphalan (BEAM) + ASCT and underwent a second randomisation to receive 1-year rituximab maintenance therapy or remain under observation. Overall, only about 50% of patients underwent SCT. An analysis was carried out on patients who failed this therapy, namely patients who failed to respond to R-ICE or R-DHAP (thus being ineligible for transplantation) in the first instance (refractory patients) and patients who relapsed immediately after transplantation or after the second randomisation.22 In the refractory patients (i.e. patients who had failed R-ICE or R-DHAP), the overall response rate (ORR) to third-line chemotherapy (including switching regimens, anthracycline-containing regimens, and acute leukaemia-type treatment) was 39%, with a CR/CR unconfirmed (CRu) rate of 27%. There was no specific recommendation in the CORAL study regarding third-line treatments; frequently, the treatments entailed a switch from R-ICE to R-DHAP or vice versa. As no difference in results was observed between treatment types, it was concluded that there was no obvious cross-resistance between the R-ICE and R-DHAP regimens. Median overall survival for the entire population was 4.4 months; however, multivariate analysis revealed that the quality of response to third-line treatment significantly impacts the overall survival. Consequently, the median overall survival was 3.7 months in patients with stable disease/progressive disease, 11.7 months in the partial response group, and 63.6 months in the CR/CRu group. Similarly, patients who were eligible for ASCT (following achievement of clinical response) demonstrated improved median survival versus patients ineligible for ASCT (11.1 versus 3.3 months).
With regard to patients who had relapsed post ASCT (n=75) where third-line treatment was at the discretion of the physician, the majority of patients switched between the R-DHAP and R-ICE regimens whenever possible. In these patients, the ORR was 44%, with a CR/CRu rate of 32%, and a small proportion of the patients, 16 of 75 (22%) patients, eventually underwent SCT.22 In multivariate analysis, achievement of response, but not transplantation itself, was a very strong positive factor, together with the International Prognostic Index. In the case of relapse post transplantation, the interval between the CORAL transplantation and the time of relapse was important. These results demonstrate that it is still possible to achieve a response in a proportion of patients with chemorefractory DLBCL, allowing for ASCT in some and the possibility of longer-term survival.
A meta-analysis of the truly refractory patients (SCHOLAR1) was carried out whereby data from the CORAL study were combined with those from the MDACC, MC/IA, and LY.12 studies.23 Eligible patients included those refractory to a first-line therapy (as defined by >4 cycles of R-CHOP-like regimens), refractory to a second-line therapy (>2 cycles of R-DHAP, R-ICE, or rituximab plus gemcitabine, dexamethasone, and cisplatin [R-GDP]), or with a relapse early (<12 months) after ASCT. A total of 529 of 635 chemorefractory patients could be evaluated for response. The ORR for the total population was 26%, with a CR rate of 8% and a partial response rate of 18%. These results emphasise the unsatisfactory results of current strategies in R/R DLBCL.
Combination and Monotherapy Treatment for Relapsed/Refractory Aggressive B-Cell Non-Hodgkin Lymphoma or Diffuse Large B-Cell Lymphoma
Current combinations that are being studied in Phase II/III studies for R/R aggressive B-cell NHL or DLBCL include regimens such as rituximab (R)-bendamustine, R-gemcitabine, and oxaliplatin (R-GEMOX), R-lenalidomide, R-ICE, and R-DHAP. In a selection of studies (Table 1a), ORRs ranged from 35–83%, while CR/CRu rates ranged from 15–58%, demonstrating that there is a proportion of patients who can achieve a clinical response.
In a selection of Phase II/III studies assessing various monotherapies in patients with R/R NHL or DLBCL ineligible for high-dose therapy (Table 1b), ORRs ranged from 19–44%, while CR/CRu rates ranged from 0–17%. Despite the limitations of between-study comparisons (e.g. low patient numbers, mixed populations, and difference in median number of prior treatment lines), these results suggest that a small proportion of patients in this setting are still able to achieve complete remission.
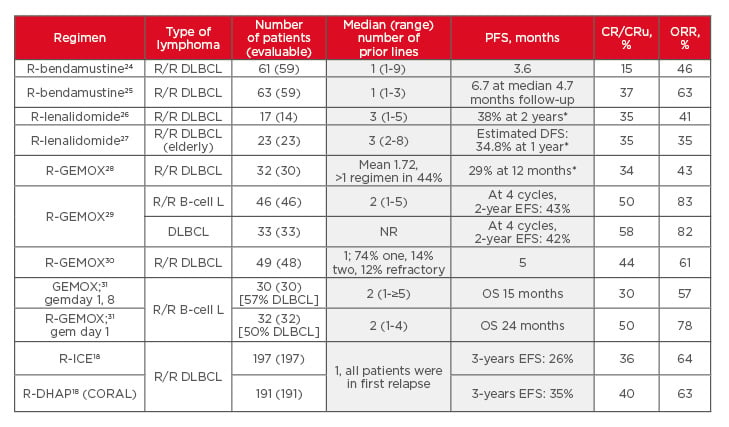
Table 1a: Selection of Phase II/III combination studies for relapsed/refractory aggressive non-Hodgkin lymphoma or diffuse large B-cell lymphoma.18,24-31
*Percentage of patients.
CR: complete response; CRu: complete response unconfirmed; DFS: disease-free survival; DHAP:
dexamethasone, cytarabine, cisplatin; DLBCL: diffuse large B-cell lymphoma; EFS: event-free survival;
gem: gemcitabine; ICE: ifosfamide, carboplatin, etoposide; L: lymphoma; NHL: non-Hodgkin lymphoma; NR: not reported; ORR: overall response rate; OS: overall survival; PFS: progression-free survival; R: rituximab; R/R: relapsed/refractory; R-GEMOX: rituximab, gemcitabine, oxaliplatin.
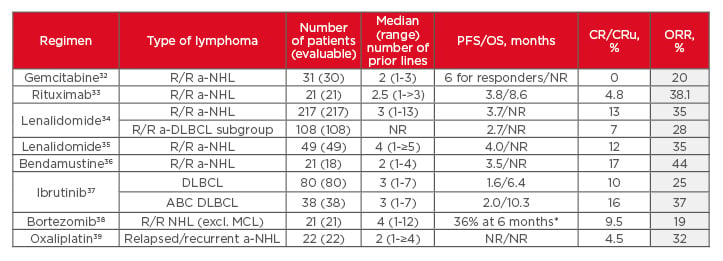
Table 1b: Selection of Phase II/III monotherapy studies for relapsed/refractory non-Hodgkin lymphoma or diffuse large B-cell lymphoma ineligible for high-dose therapy.32-39
*Percentage of patients
ABC: activated B-cell; a-DLBCL: aggressive diffuse large B-cell lymphoma; a-NHL: aggressive non-Hodgkin lymphoma; CR: complete response; CRu: complete response (unconfirmed); DLBCL: diffuse large B-cell lymphoma; MCL: mantle cell lymphoma; NHL: non-Hodgkin lymphoma; NR: not reported; ORR: overall
response rate; OS: overall survival; PFS: progression-free survival; R/R: relapsed/refractory.
In summary, achievement of a complete response in the setting of R/R lymphoma remains a positive prognostic factor. Polychemotherapy is usually preferred for transplant-eligible patients and monotherapies are usually reserved for other patients (with pixantrone being a good option with non-negligible efficacy). Currently awaited are the results of ongoing studies evaluating the incorporation of pixantrone into multiple-drug regimens and potentially as a bridge to transplantation.
Pixantrone for Third and Fourth-Line Treatment of Relapsed/Refractory Non-Hodgkin B-Cell Lymphoma
Professor Ruth Pettengell
Pixantrone: Indication and Guideline Recommendations
Pixantrone is the only drug that has evidence from Phase III randomised controlled trials (RCT) and is licensed as a single agent for the third and fourth-line treatment of multiple R/R-aggressive B-cell NHL within Europe. Conditional marketing authorisation was granted in the European Union (EU) (May 2012) with the specific obligation to conduct the PIX306 study, a Phase III RCT.13 The use of pixantrone is mentioned in the European Society for Medical Oncology (ESMO) guidelines,1 as well as in many national guidelines, including the National Institute for Health and Care Excellence (NICE) guidelines.40
Mechanism of Action and Unique Chemical Structure of Pixantrone
Pixantrone is a novel aza-anthracenedione with a mechanism of action that is distinct from that of anthracyclines and anthracenediones, such as doxorubicin and mitoxantrone. Classical anthracyclines work by inhibiting DNA topoisomerase 2 alpha, resulting in DNA damage and relaying a signal through the p53 cell cycle-dependent pathway, ultimately resulting in apoptosis. In contrast, pixantrone works predominantly as a DNA intercalator (Figure 2); the resultant chromosome bridges and micro and multi-nucleation eventually lead to abnormal mitosis and cell death.41
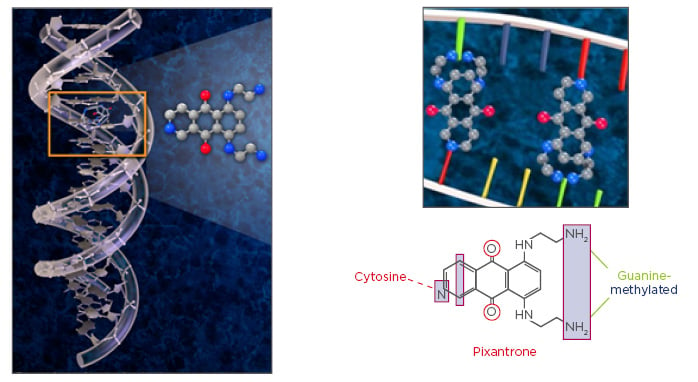
Figure 2: Pixantrone has a unique mechanism of action.
© Les Laboratoires Servier, 2017 (published with permission).
The chemical structure of pixantrone also confers unique properties in terms of safety. Compared with anthracyclines, pixantrone lacks an iron-binding site.42,43 It therefore does not form toxic drug-metal complexes,43 which limits the release of reactive oxygen species and concomitant cardiac toxicity. Additionally, alcohol metabolites of pixantrone accumulate to a lesser degree in cardiac tissue14 compared with doxorubicin, and furthermore, pixantrone appears to be able to displace the previously accumulated doxorubicin alcohol metabolites. As such, pixantrone does not add to the existing toxicity in patients previously treated with anthracyclines. In summary, pixantrone lacks reduction–oxidation (i.e. redox) activity and inhibits doxorubicinol formation in human myocardium.44
Reduced Cardiotoxicity of Pixantrone in the Clinic Compared with Doxorubicin
Results from RCTs have demonstrated that the findings of preclinical studies have carried through to clinical practice. In the first trial, a comparison of a pixantrone-based regimen (R-CPOP: rituximab with cyclophosphamide, pixantrone, vincristine, and prednisolone) was compared with doxorubicin-based therapy (R-CHOP) for first-line therapy in DLBCL patients.45 Compared with the R-CHOP arm, a lower percentage of R-CPOP-treated patients had a decrease from baseline in left ventricular ejection fraction (decreases of ≥10% to <50% [27% versus 15%, respectively]; ≥15% [32% versus 17%]; ≥20% [17.5% versus 2%]). In addition, a higher percentage of R-CHOP-treated patients had a significant increase in troponin T (33% versus 7%, respectively). These results demonstrate that indicators for cardiac toxicity are favourable for pixantrone even in the first-line setting. In terms of clinical evidence of cardiotoxicity, 6% of patients in the R-CHOP arm experienced Grade 3 congestive heart failure compared with none in the R-CPOP group.
PIX301 was a multicentre, randomised, active-controlled study evaluating the efficacy and safety of pixantrone as a single-agent therapy in the management of patients with aggressive R/R-NHL (73% with DLBCL) who had received at least two prior therapies and had adequate cardiac function (left ventricular ejection fraction: ≥50%) at time of study entry.14 Patients were randomised 1:1 to either pixantrone (50 mg/m2 on Days 1, 8, and 15, every 28 days, ≤6 cycles) or to available active comparators in the EU and USA (physician’s choice, used at standard therapeutic doses and schedules). Patients had a maximum prior cumulative dose of anthracyclines of 450 mg/m2 or equivalent, indicating that patients were heavily pretreated upon study entry. Importantly, patients had to have had at least a 6-month response to previous anthracycline-based chemotherapy and therefore this study excluded primary refractory patients. Baseline characteristics were well-balanced between the pixantrone and the comparator arm except for cardiac toxicity; 3 patients in the pixantrone group had a history of congestive heart failure and 2 had cardiomyopathy (no history of cardiac problems was reported in the comparator arm); hence, it is difficult to interpret the cardiac outcomes in the study.
A significant difference was observed between the pixantrone and comparator arms in terms of CR/CRu rates at the end of study (24% versus 7%, respectively); the median duration of CR/CRu (9.6 versus 4 months, respectively) and progression-free survival (5.3 versus 2.6 months, with a hazard ratio of 0.6).
In terms of pixantrone safety, the predominant toxicity (Grade 3/4) was haematological events, with neutropenia in particular (41% versus 19% in the comparator arm). However, this did not translate into a great increase in febrile neutropenia (7% versus 3% in the comparator arm). In this study, granulocyte colony-stimulating factor (G-CSF) use was allowed; however, as no specific guidelines on the use of G-CSF were provided in the study protocol, few patients received G-CSF as recommended. The rate of anaemia was higher in the comparator arm (13% versus 6% in the pixantrone arm) while thrombocytopenia rates were comparable in both arms (12% and 10% in the pixantrone and comparator arms, respectively). However, it is difficult to make comparisons with the comparator group as the median number of drug cycles was different in the pixantrone and comparator arms (four versus three cycles, respectively), and there was a discrepancy in the frequency of blood tests between the two groups due to the specific treatment administration schedule in the pixantrone arm. In terms of non-haematological toxicity, rates of events were low. Cardiac events were observed in the pixantrone group but as mentioned above, interpretation of these results is difficult due to the imbalance in cardiac abnormalities at baseline; nonetheless, the majority of the cardiac events were asymptomatic and reversible, having resolved by the end of the study.
PIX306 Study
A post hoc analysis of the PIX301 study showed that the efficacy of pixantrone versus comparator was independent of prior rituximab therapy.15 Pixantrone has conditional marketing approval in the EU based on the results of the PIX301 study.13 The PIX306 study design was endorsed by the EMA as a post-marketing commitment required to convert to full marketing approval. The study aimed to confirm the efficacy of pixantrone in patients progressing after prior rituximab-containing regimens, as well as to provide information to guide treatment of transplant-ineligible relapsed lymphoma patients. Gemcitabine is used in the comparator arm (in combination with rituximab), as it was commonly in use as a single agent/doublet at the time of the study design. Additionally (unlike PIX301), patients only had to have had a 12-week response to a previous line of therapy and there were no specific requirements in terms of prior anthracycline use. To date, 291 (out of a planned recruitment of 320) patients have been recruited and preliminary results are expected in summer 2018.
Patient Cases
Doctor Amjad Hayat and Professor Kai Hübel
Dr Hayat and Prof Hübel presented case studies of patients with relapsed DLBCL who received pixantrone monotherapy. The cases demonstrated that treatment with pixantrone is manageable in older patients, and is an option in heavily pretreated patients not responding to chemotherapy.
PANEL DISCUSSION
Q: With the availability of a vast range of novel treatments for R/R DLBCL, how do you choose which to use and which patients should receive these treatments? Please comment on the toxicity profile of pixantrone in comparison with some of the novel agents.
Prof Zinzani replied that, for the most part, the novel agents are still in clinical trials; generally, pixantrone was prescribed after the second-line treatment for patients who were ineligible for SCT, due to lack of cardiac toxicity and the higher clinical response compared with other biological agents.
The major toxicities with pixantrone are neutropenia and cytopenia; however, these toxicities could be managed by limiting the dosing schedule to Days 1 and 8 (and not Day 15). Satisfactory (clinical) results were obtained with this change in schedule.
Q: How is neutropenia managed in response to chemotherapy and is neutropenia a big concern?
Prof Van Den Neste replied that one of the major considerations when choosing a treatment regimen is the rate of Grade 3/4 neutropenia, as well as the rates of infection, infestation, and febrile neutropenia. Another consideration is whether the neutropenia can be treated with growth factors (GFs) and whether these GFs are available/ reimbursable. Pixantrone had a relatively favourable profile, even though it is associated with significant haematological toxicities, because of the low rate of infection. The issue would be how to give GFs in combination with weekly pixantrone infusions (unlike DHAP or ICE).
Prof Pettengell added that it would be important for GFs to be started within 72 hours of chemotherapy and ideally stopped 24 hours before the next cytotoxic chemotherapy. She also mentioned that she would be comfortable treating with G-CSF between pixantrone injections, as it was not a cell-cycle specific agent.
Q: Which patients in your practice would you choose to treat with pixantrone?
Prof Van Den Neste replied that he would select patients that were ‘in between’, meaning patients who were not eligible or had relapsed after ASCT, but who were still able to cope with frequent hospital visits, had the need for antibiotics in case of neutropenia, as well as being able to cope with febrile neutropenia. As haematological adverse events are an issue, the patients would still have to be selected with caution (perhaps with the use of comorbidity scoring methods).
SUMMARY AND CONCLUSION
Within clinical practice, a significant proportion (about 75%) of patients with R/R aggressive B-cell NHL who fail first-line therapy will go on to potentially having third or fourth-line therapy. There is no accepted standard of care for third or fourth-line treatment. Pixantrone monotherapy has significantly greater efficacy than active comparator agents in these patients and can be used for patients with significant prior anthracycline exposure. It has a predictable and manageable safety profile, and in addition, a flexible dosing schedule can be utilised to manage toxicities. Clinical trials of pixantrone combined with other standard regimens are ongoing.







