Meeting Summary
This symposium was dedicated to discussing BCR-ABL-positive chronic myeloid leukaemia (CML) and Philadelphia-positive acute lymphoblastic leukaemia (Ph+ALL). Prof Baccarani opened the symposium, highlighting the recent improvements in survival in patients with BCR-ABL-positive CML and Ph+ALL. Dr de Lavallade discussed the role of mutational analyses as part of molecular monitoring, including the use of next-generation sequencing (NGS) to assess BCR-ABL mutation status and to detect low-frequency mutations. Dr Rea reviewed treatment options for CML with tyrosine kinase inhibitors (TKI) in the second and third-line treatment settings. The session concluded with Dr Martinelli presenting mutational burden in Ph+ALL patients and treatment options for these patients, in particular, with ponatinib, emphasising the importance of early treatment initiation.
Welcome and Introduction
Professor Michele Baccarani
Treatment of BCR-ABL-positive leukaemias is a success story. Ten-year survival in patients with BCR-ABL-positive CML has improved over the last 20 years, from <10% to >80%, with a number of functional cures and treatment-free remissions.1 Ph+ALL remains more challenging to treat; nevertheless, 5-year survival has reached up to 50% for a condition that used to be fatal for nearly all patients.2 These improvements in treatment include a significant reduction in toxicity, and achievement of cures in some patients.
Going forward, the key challenge in Ph+ALL will be to develop combination therapies to keep improving survival, whereas the aims for CML will include continuing optimisation of therapeutic strategies and improving quality of life, particularly in second and third-line settings. Therapies should be individually tailored, as well as considering cost and toxicity profiles. In optimal responders of patients with CML, advanced age and particular comorbidities, such as cerebrovascular disease, heart failure, psychiatric disorders, Alzheimer’s disease, or Parkinson’s disease, may have more impact on patients’ survival and quality of life than the CML itself. The current version of the European Leukemianet (ELN) recommendations was published in 2013, and updated recommendations are anticipated, which will provide further guidance on optimising treatment and management of these patients.
The Evolving Role of Molecular Monitoring
Doctor Hugues de Lavallade
Mutations in the BCR-ABL fusion gene, which occur most frequently in the kinase domain (KD), are important drivers of TKI resistance in CML. Early detection of BCR-ABL mutation status is therefore important, and may help to reverse the poor prognosis associated with these mutations through switching to alternative TKI.
Current ELN guidelines1 recommend molecular testing every 3 months until the patient reaches major molecular response (MMR). This is being implemented in clinical practice, where the frequency of molecular testing at 3, 6, and 12 months has increased over the last 5 years.3 Mutation testing should be done in case of treatment failure, when switching from one TKI to another, and in case of loss of MMR or suboptimal response, as per current guidelines.1 Whether there is a role for mutation testing as a routine surveillance measure remains controversial. There is currently no guidance on how frequently a patient who has tested negative for mutation should be monitored;1 however, this could be helpful in the future to encourage early detection of mutations.
Although early identification of BCR-ABL KD mutations may help to reverse the associated poor prognosis, in most instances, Sanger sequencing does not detect mutations occurring at <20% frequency. Multiplex mass spectrometry was among the first techniques used to detect low-level mutations, pioneered by an Australian group led by Tim Hughes. The technique detected 31 mutations at frequencies as low as 1%, and showed that the T315I mutation conferred resistance to TKI therapy, eventually leading to loss of treatment response.4
Ultra-deep NGS techniques provide another, more widely available, platform for the identification of low-burden mutations. The mutations identified by NGS at >20% frequency could consistently be confirmed by Sanger sequencing, whereas those present at <16% frequency could not be found with the less sensitive technique.5 In addition, NGS allows accurate quantification of mutation frequency.5
One pitfall of NGS is the risk of artefacts in the sequencing results, leading to false-positive identification of mutations.6 This is a particular issue when two rounds of polymerase chain reaction (PCR) are used for gene amplification (the nested PCR technique).5 Under these experimental conditions, the frequency of false-positive results was reported at ≤3% according to screening of 27 healthy control patients.5 A single round of PCR should therefore be used to amplify complementary DNA for NGS.
Resistance to a TKI may be impacted by the type of BCR-ABL mutation present. In particular, it is important to distinguish between whether a mutation is a driver mutation (Figure 1A) or a passenger mutation (Figure 1B). This may be clearer for some mutations than others. The E459K mutation (Figure 1A), for example, is clearly driving treatment resistance and progression to the blastic phase. This can be seen because the patient loses complete cytogenetic response (CCyR) once the clone harbouring the mutation reaches very high frequency, leading to further transformation to the blastic phase.7 In contrast, a passenger mutation such as M244V in Figure 1B is not conferring resistance to TKI therapy, and the number of leukaemic cells would reduce with the reduction of BCR-ABL/ABL transcript ratio.7 However, the presence of a mutation is, per se, a marker of genomic instability and according to current ELN recommendations,1 any emerging mutation in first-line treatment and any new emerging mutation in second-line treatment should prompt a change in therapy.
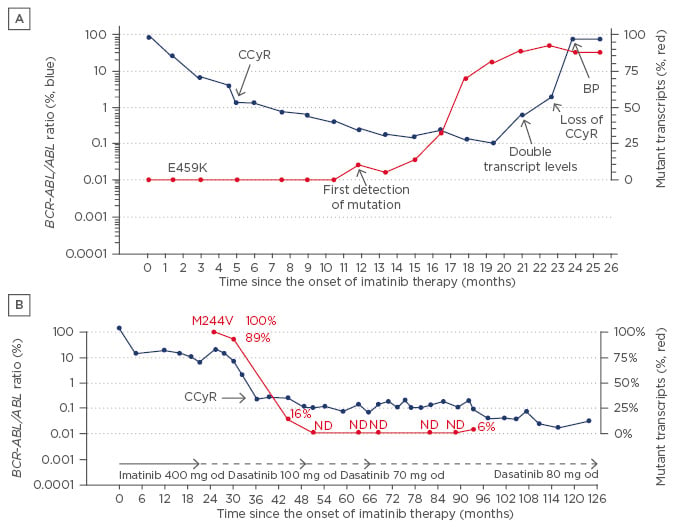
Figure 1: Identification of driver mutations (A) or passenger mutations (B) in chronic myeloid leukaemia. BCR-ABL/ABL transcript ratiocan be seen in blue (left-hand Y axis; A, B), and the percentage of the mutated clone can be seen in red (right-hand Y axis; A, B).
CCyR: complete cytogenetic response; od: once daily; BP: blood pressure; ND: no difference.
Adapted from Kizilors and de Lavallade, unpublished data.
To determine the incidence and prognostic significance of KD mutations, irrespective of the patients’ response to TKI, a retrospective, systematic screening was carried out in a population of patients with CML, using NGS.5 The study identified several known KD variants in the overall patient population.5 Overall, mutations were detected in 25 of 121 patients screened, including 15 of 38 (39%) patients who failed TKI therapy, 6 of 28 (21%) patients who had suboptimal response, 2 of 15 (13%) patients who had dose interruptions, and 2 of 40 (5%) patients who had optimal response.5 Patients with mutations had significantly worse progression-free survival than those without mutations.5 Forty-one patients had samples available at the 3-month time point, with 20 patients having a high BCR-ABL transcript level (>10%), showing that the population is skewed towards those with poor prognosis. Mutations were identified at 3 months in 4 patients, all of whom progressed to the accelerated or blastic phase after the mutation was detected.5
Overall, it is important to monitor mutation status and frequency, even if the mutation does not confer resistance to a TKI, because it can be indicative of genomic instability. Standardisation of NGS protocols will be of value to further improve the reliability of NGS and to avoid the reporting of artefacts. Dr de Lavallade’s group is planning prospective studies in the UK to look at the clinical impact of early treatment-switching to prevent loss of response for patients with mutations.
Factors Affecting Clinical Decision-Making in Refractory and Relapsed Chronic-Phase Chronic Myeloid Leukaemia Patients
Doctor Delphine Rea
Relapsed or refractory disease refers to the development of resistance, and is associated with a higher risk of progression to advanced-stage CML and worse overall survival. The ELN 2013 recommendations categorise resistance into two groups: primary resistance (refractoriness, i.e. lack of efficacy from treatment initiation), and secondary resistance (loss of treatment efficacy after an initial response). Treatment options for relapsed/ refractory CML include second or third-generation TKI, or allogeneic stem-cell transplantation (SCT) for eligible patients.1
Resistance is mediated through several mechanisms, as outlined in Figure 2, which relate to the properties or dose of the drug (e.g. pharmacokinetics/ pharmacodynamics, impaired import/export, lack of adherence, or inappropriate dose reduction); oncogenic signals in addition to BCR-ABL signalling; and BCR-ABL-related signals, including KD mutations.8-11
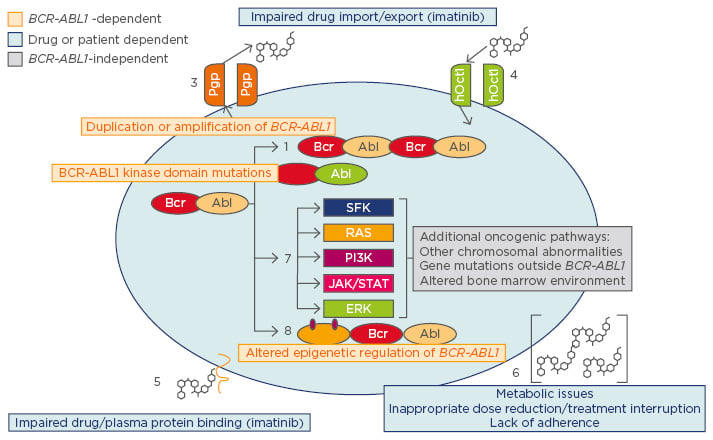
Figure 2: Mechanisms of resistance to TKI.
TKI: tyrosine kinase inhibitors.
Adapted from Apperley,8 Bixby and Talpaz,9 Eiring and Deininger,10 Hochhaus et al.11
More than 100 mutations have been identified across the BCR–ABL KD, although mutations have also been identified in the P-loop, the activation loop, and the ATP binding site/gatekeeper site. Resistance frequently occurs to the first-generation TKI imatinib; most mutations can be effectively treated with the second-generation TKI dasatinib, nilotinib, and bosutinib. The exception is T315I, which prevents binding of all approved first and second-generation TKIs to BCR-ABL. The third-generation TKI ponatinib was developed specifically to bind to BCR-ABL harbouring the T315I mutation;12,13 its efficacy has been shown in in vitro kinase and cellular proliferation assays.10,14,15,16 Ponatinib is also effective against all other published single BCR-ABL mutations and has activity against other kinases, which may impact tolerability, but also may provide an advantage by targeting BCR-ABL-independent pathways.
The ENESTnd study17,18 assessed the scope of resistance in chronic-phase CML following first-line therapy; 15.5% patients were resistant to imatinib at 18 months versus 4.2% and 3.5% with nilotinib 300 mg and 400 mg, respectively.17,18 A higher Sokal score predicts greater probability of resistance to first-line therapy, especially for imatinib therapy, but this is also the case for nilotinib-treated patients.17,18
Registration studies have shown that ˜50% of patients treated with second-line dasatinib, nilotinib, or bosutinib do not achieve CCyR.19-22 Ponatinib could increase the rate of salvage in the second-line setting; however, more data are required to address this question. For patients who fail first-line treatment with a second-generation TKI, ELN recommendations for second-line therapy are an alternate TKI other than imatinib.1 However, there have been no clinical studies addressing the best treatment option in this situation. A case series23 of 10 patients who failed first-line dasatinib or nilotinib in the setting of primary or secondary resistance, including 8 patients without a T315I mutation, received second-line ponatinib between 45 and 30 mg daily. Six of the eight patients gained an optimal response and all patients reached a MMR, whereas some gained a deeper molecular response.
The prospects of achieving deep and sustained responses deteriorate in the third-line setting for patients who have failed two previous therapies. In this situation, the 1-year mortality rate due to CML increased to 4.25%, which is much higher than the background rate of death.24 ELN 2013 recommendations state that any of the remaining TKIs, or allogeneic SCT in eligible patients, may be considered.1 There are no direct comparisons of the second-generation TKIs in the third-line setting, but a meta-analysis comparing the available evidence showed a 22–26% response with secondgeneration TKI. In comparison, the PACE trial showed a response rate with ponatinib of 60% for patients harbouring T315I and a 52% response rate for patients without the T315I.25,26 Indeed, 4-year data from the PACE trial showed a CCyR rate of 54%, a MMR of 39%, and some deep molecular responses, which is of note in this heavily pre-treated relapsed/refractory patient population. The CCyR was achieved in patients with T315I mutation only (74%), with other BCR-ABL mutations (57%), or without a mutation (49%), and the response was maintained after 4 years in 61% patients; achieving a sustained response is a key goal in the treatment of this patient population to avoid transformation to the accelerated or blastic phase of CML.26
Furthermore, in a post hoc, retrospective, indirect comparison of overall survival data of T315I positive CP-CML patients in the PACE trial with those receiving allogeneic SCT as reported to the European Bone Marrow Transplant registry, the overall survival at 4 years was better with ponatinib than with SCT.27 Thus, ponatinib alone seems to be a valuable alternative to transplantation for prolonging survival in this patient population. It was noted that 5-year data from the PACE study would be presented at the congress on Saturday 24th June by Cortes et al.28
PACE safety results at 4 years were consistent with the safety profile across the ponatinib clinical programme: Adverse events occurring in ≥20% patients affected the skin, the gastrointestinal system, the musculoskeletal system, and the pancreas; however, the major issue is cardiovascular (CV) safety, e.g. 13% patients experienced hypertension Grade 3 or 4 events with ponatinib therapy. Eleven percent of patients experienced serious CV adverse events, in particular, serious arterial occlusive events (AOE).26 The CV risk is greatest with ponatinib in patients with CV risk factors at baseline, such as diabetes or hypertension, and in those taking higher ponatinib doses. Reducing ponatinib dose once patients have achieved major cytogenetic response may significantly reduce the risk of CV events, as seen in the PACE trial where dose reduction was instructed in October 2013 to mitigate AOE.10,26 Additional data from a long-term follow-up of this clinical trial have demonstrated that the exposure-adjusted incidence of AOE has not increased over time, and responses were maintained in the vast majority of patients after reducing the dose to 30 or 15 mg per day. Findings from patients who have undergone dose reduction after achieving a major cytogenetic response provide reassurance that ponatinib continues to be effective in maintaining this effect when a lower dose is taken, and the ponatinib summary of product characteristics now recommends considering dose reduction to 15 mg for patients who have achieved major cytogenetic response.29 However, the cytogenetic response should be closely monitored upon dose-reduction to ensure that the response is maintained, as there is currently no formal dose-response analysis available. Overall, ponatinib is highly effective in the third and subsequent-line setting, and may be the best choice after failure of first-line second-generation TKI. Allosteric inhibitors are currently in development and, going forward, may provide an additional treatment option for this challenging patient population.
Current Challenges, New Insights, and Future Directions in Philadelphia-positive Acute Lymphoblastic Leukaemia
Doctor Giovanni Martinelli
The median 5-year survival for adult patients with ALL remains at approximately 40%,2,30 so improving treatment for patients with ALL, particularly Ph+ALL, is one of the key unmet medical needs in leukaemia. Mutation analysis by the sensitive denaturing high-performance liquid chromatography (D-HPLC) technique in Ph+ALL patients resistant to imatinib revealed a high frequency of mutations: approximately 70% of patients were positive for BCR-ABL KD mutations (90% with single mutation, 10% with two or three single mutations). The gatekeeper mutation T315I was the most frequent mutation with close to 40% frequency. In patients resistant to first and second-generation TKIs, the number of T315I positive patients increased to 65%. Additionally, the proportion of patients with difficult-to-treat multiple mutations increased significantly (42% with single mutations versus 58% with multiple mutations). Of interest, half the patients with multiple mutations revealed compound mutations corresponding to two mutations on the same strand of DNA.15 As these mutations are associated with high resistance to nearly all available TKIs, it seems of utmost importance to treat these patients with a highly effective TKI in an early treatment line to avoid expansion of resistant clones harbouring multiple point mutations.
This resistance pattern is relevant to a series of patients treated in clinical trials with upfront dasatinib and steroids or low-intensity chemotherapy, in which approximately 70–75% of patients who were treated first-line with dasatinib developed a T315I mutation that was selected for by TKI therapy.31,32 A retrospective longitudinal analysis of 34 patients showed that early detection of the mutation is possible via NGS techniques, ≤8 weeks prior to relapse,33 so there is time to change treatment before relapse occurs. Frequent screening of mutations with NGS is particularly important in ALL because it is less genetically stable than CML, with many mutations occurring in a polyclonal situation; TKI therapy is likely to select for a particular mutation such as T315I.
Because the majority of patients are sensitive to TKI inhibitors, but are still susceptible to the emergence of new clonal mutations, a Phase II study of ponatinib in combination with conventional chemotherapy using hyper-CVAD (cyclophosphamide + vincristine + doxorubicin + dexamethasone) was performed in 36 patients with Ph+ALL.34 Initially all patients started with ponatinib 45 mg/day. Following a protocol amendment, the ponatinib dose was reduced in Cycle 2 to 30 mg for patients not in complete molecular response (CMR) or to 15 mg for patients in CMR. Study patients received intrathecal central nervous system prophylaxis. All 36 patients achieved complete remission and CCyR, whereas 95% achieved MMR and 70% achieved CMR. All responses were ongoing after a median follow-up of 16 months. Event-free survival at 2 years was 81% (95% confidence interval: 64–90). Overall, these data suggest that this hyper-CVAD/ ponatinib combination regimen is likely to be very effective for the treatment of Ph+ALL. It was noted that an update from this study would be presented as a poster at the congress on Saturday 24th June by Short et al.35
Older or frailer patients with Ph+ALL may not be suitable for aggressive chemotherapy or SCT. The GIMEMA clinical trial, LAL1811, is a Phase II study in patients of more than 60 years old or unfit for a programme of intensive chemotherapy and SCT. The aim is to explore the impact of front-line therapy with ponatinib. The primary endpoint aim is for 75% of participants to reach a complete haematological remission after 6 months.36 The study is currently closing and the results are anticipated soon.
Why is ponatinib more efficacious than second-generation TKI? Potential mechanisms for this efficacy are shown in Figure 3. In addition to its inhibition of BCR-ABL, ponatinib inhibits HCK, a leukaemic stem-cell gene that was previously predicted to be a therapeutic target for chemotherapy-resistant human leukaemia stem cells, and FLT3, which may be mutated or overexpressed in Ph+ and Ph- leukaemias.37
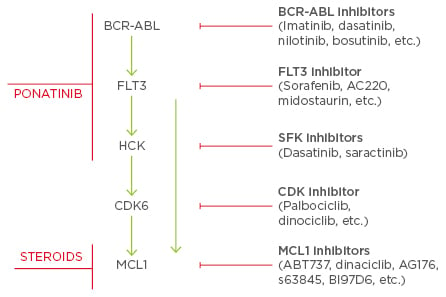
Figure 3: Mechanisms of action for ponatinib and steroids in Philadelphia-positive acute
lymphoblastic leukaemia.
SFK: Src family kinases; FLT3: FMS-like tyrosine kinase-3; HCK: haematopoietic cell kinase; CDK:
cyclin-dependent kinases; MCL1: induced myeloid leukaemia cell differentiation protein; Ph+ALL:
philadelphia-positive acute lymphoblastic leukaemia.
Adapted from National Cancer Institute,38 Aleem et al.,39 Beekman and Howell,40 Konig and Levis,41 Kotschy et al.,42 Poh et al.44
In Ph+ALL, many other proteins may be mutated, protecting the cell against death from cytotoxic therapies. For example, overexpression of proteins such as BCL2 or MCL1 may prevent activation of apoptosis pathways following DNA damage induced by conventional chemotherapies. Ponatinib appears to target ≥3 driver mutations in Ph+ALL, providing additional benefit compared with second-generation TKI. In addition, in the GIMEMA programme, the use of steroids serendipitously added inhibition of the driver gene MCL1, further helping to improve treatment outcomes.38-44
Although other drugs are being developed for the treatment of Ph+ leukaemias, ponatinib has been tested in Ph+ALL, has a favourable safety profile, and is efficacious in fit patients in combination with aggressive chemotherapy regimens, or in patients >60 years old or who are unfit for conventional chemotherapy. The treatment was associated with a relatively high quality of life in this population, and early complications due to therapy were not experienced. Most importantly, ponatinib in combination with steroids was associated with a high rate of minimal residual disease negativity and fast transcript reduction.
Closure
Doctor Eduardo Olavarria
Dr Olavarria closed the session, thanking the speakers, Prof Baccarani, and the audience for their contribution, and Incyte Biosciences International for their support in organising the meeting. He highlighted the key discussions on the importance of molecular monitoring, recommendations for use of NGS techniques, and the importance of treatment with ponatinib early before the appearance of multiple clones in both CML and Ph+ALL.







