Meeting Summary
Multiple myeloma (MM), characterised by the clonal proliferation of malignant plasma cells, results in the overproduction of monoclonal immunoglobulins.1 Genetic heterogeneity of these clones confers treatment resistance and contributes to disease progression. Therefore, the use of combination therapies with different mechanisms of action can target the maximum number of clones simultaneously and may achieve long-term disease control.2 Current therapeutic strategies, such as chemotherapy, radiotherapy, proteasome inhibitors (PI), immunomodulatory drugs (IMiD), monoclonal antibodies, and autologous/allogeneic stem cell transplantation have resulted in improved outcomes for MM patients. However, these therapies rarely induce long-lasting complete remissions, and patients frequently develop resistance to treatments. As such, the search for novel treatment strategies, including personalised immunotherapies, is ongoing to overcome resistance and improve patient survival.
Steady Stream and Changing Seas in Myeloma
Professor Jesús San Miguel
Treatment of MM requires a multifaceted approach using a combination of therapies targeting the several pathophysiological pathways involved in the disease. PI are a key backbone therapy in the treatment of MM by targeting an integral pathophysiological pathway in myeloma cells and the bone marrow microenvironment simultaneously.1 However, clonal heterogeneity means that combination therapy is needed to tackle the multiple pathogenic pathways inherent in MM. PI can be synergistically combined with IMiD, another backbone treatment, as well as monoclonal antibodies to improve outcomes.
Overactivation of the ubiquitin proteasome pathway, which maintains cellular homeostasis, results in an anti-apoptotic state and is a hallmark of MM.3,4 Inhibition of the pathway leads to accumulation of misfolded and regulatory proteins triggering endoplasmic reticulum stress, activation of the unfolded protein response, and apoptosis.3,5 Furthermore, treatment with PI suppresses the NF-κB pathway, downregulating anti-apoptotic factors and promoting apoptosis of myeloma cells.5 Additionally, in the bone marrow microenvironment, proteasome inhibition downregulates cytokine secretion; cell proliferation, adhesion, and migration; and decreases tumour angiogenesis.1
IMiD exert potent anti-myeloma activity by stimulation of apoptosis and inhibition of angiogenesis, adhesion, and cytokine circuits within the bone marrow microenvironment, as well as enhancement of anti-tumour immune responses through T cell and natural killer cell alterations.6 Monoclonal antibodies target cell surface antigens to induce apoptosis by alterations in intracellular signalling, growth factor receptor inhibition, adhesion molecule inhibition, as well as direct antibody-dependent cellular toxicity resulting in enhanced myeloma cell death.7,8 Current treatment regimens, as recommended by the European Society for Medical Oncology (ESMO) guidelines, combine PI with IMiD and/or monoclonal antibodies with corticosteroids to provide synergistic treatment options that target the multiple pathogenic pathways present in MM.9
Lenalidomide, an IMiD, and dexamethasone (collectively termed ‘Rd’) is an established backbone treatment for relapsed MM patients. Evidence from the Phase III ASPIRE study indicates that the addition of carfilzomib, a PI, to lenalidomide and dexamethasone (KRd) significantly improves patient outcomes.10,11 Median progression-free survival (PFS) was significantly improved in the KRd group (26.3 months) compared to the Rd group (17.6 months).11 In addition, median overall survival (OS) was 48.3 months (95% confidence interval [CI]: 42.4–52.8) for KRd versus 40.4 months (95% CI: 33.6–44.4) for Rd.10
Another treatment approach includes the use of monoclonal antibodies in the treatment regimen. Findings from the Phase III CASTOR study indicated that the addition of daratumumab, a human IgG monoclonal antibody targeting CD38 proteins on myeloma cells, to a PI and dexamethasone backbone provides significant improvements. Patients with relapsed/refractory MM (RRMM) were treated with a combination of bortezomib and dexamethasone (Vd) or a triple combination with daratumumab, bortezomib, and dexamethasone (DVd). Treatment with DVd resulted in significantly longer PFS than Vd alone (16.7 versus 7.1 months), demonstrating the benefits of monoclonal antibody treatment in MM.12 The combination of daratumumab with a second-generation PI, carfilzomib, is currently being tested in a Phase III randomised CANDOR study.13
Recently, concepts such as early detection and intervention, eradication of resistant clones, cytogenetic risk, and personalised medicine have altered the approach to treatment. Evidence from the Phase III QuiRedex study highlights the importance of early detection and treatment with Rd in high-risk smouldering MM patients. Early treatment with Rd significantly delayed the time to progression to myeloma compared to the observation group (not reached versus 23 months).14,15 Similarly, the CESAR trial, in which high-risk smouldering MM patients were treated with KRd both before autologous stem cell transplantation (ASCT) and post-transplant in the consolidation phase, demonstrated similar improvements: 93% of patients were progression-free at 32 months. Significant improvements in response rates including minimal residual disease (MRD)-negativity, an established prognostic marker, were observed throughout the treatment sequence. The proportion of patients achieving a complete response (CR) or better during the induction, ASCT, and consolidation phase were 42%, 64%, and 76%, respectively.16 Importantly, patients achieving durable MRD-negative status were less likely to experience a relapse compared to MRD-positive patients. This is supported by results from the PETHEMA/GEM2010MAS65 study demonstrating improved survival rates in patients that achieved MRD-negativity irrespective of age or cytogenic risk.17 PFS rates at 3 years were 92%, 70%, 54%, and 44% for patients who were MRD-negative (<10-6), MRD-positive (10-6), MRD-positive (10-5), and MRD-positive (≥10-4), respectively, with only 3% of patients relapsing.17
To improve outcomes and overcome treatment resistance, the foundations of disease management have evolved to include a new generation of PI and IMiD, together with monoclonal antibodies. Additionally, recent advances in novel immunotherapy development and the growing understanding of MM pathophysiology are leading to personalised medicine.
An Uphill Battle: Overcoming Treatment Resistance
Professor Katja Weisel
Treating MM is a long-term endeavour requiring a range of therapeutic strategies as the nature of the disease changes over time. The front-line therapy for newly diagnosed MM is evolving with new combinations of PI, IMiD, monoclonal antibodies, and corticosteroids. Extended duration of highly active combinations in early lines is resulting in increased treatment resistance as the disease relapses, which is becoming an important consideration in clinical practice. Development of treatment resistance is multifaceted, including adaptation of malignant cells and alteration of the microenvironment. Overcoming treatment resistance can be achieved by targeting either intracellular or extracellular pathophysiological pathways with novel treatments.
The proportion of lenalidomide-refractory patients in early-RRMM combination trials is currently underrepresented. Recent Phase III studies such as CASTOR, ENDEAVOR, ARROW, OPTIMISMM, and ELOQUENT-3 show a growing trend in the proportion of lenalidomide-refractory patients with 24%, 24%, 75%, 71%, and 90% identified, respectively, in their active arms (Figure 1).18-22
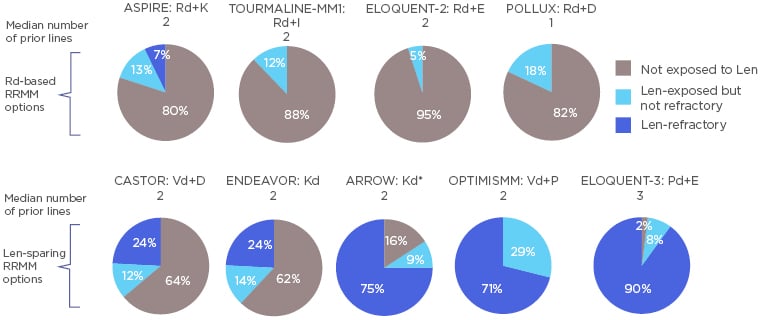
Figure 1: Proportion of patients in relapsed/refractory multiple myeloma drug combination trials exposed to lenalidomide but not refractory, and lenalidomide-refractory. *Kd patients from both study arms are represented.
D: daratumumab; d: dexamethasone; E: elotuzumab; I: isatuximab; IMiD: immunomodulatory drug; K: carfilzomib; P: pomalidomide; R/Len: lenalidomide; RRMM: relapsed/refractory multiple myeloma; V: bortezomib.
These studies provide evidence on treatment options for the emerging lenalidomide-refractory patient population by excluding lenalidomide from the treatment combinations.
One such trial that represents a lenalidomide-sparing option is the Phase III ARROW study of once-weekly Kd with carfilzomib at 70 mg/m2 (Kd70), or twice-weekly Kd with carfilzomib at 27 mg/m2 (Kd27) in RRMM. Of patients enrolled in the ARROW trial, 75% were lenalidomide-refractory.20 In the overall population, Kd70 once-weekly and Kd27 twice-weekly treatment conferred a median PFS of 11.2 months and 7.6 months, respectively. To address the unmet need of treatment for lenalidomide-refractory patients, a post-hoc meta-analysis of 1,107 Kd-treated patients from the ARROW, ENDEAVOR, and CHAMPION-1 studies was performed to evaluate efficacy and safety of Kd in patients who were previously exposed, or refractory, to lenalidomide.23 Median PFS of lenalidomide-refractory patients with one prior line of treatment was 15.6 months in both lenalidomide-refractory and non-refractory subgroups.
Lenalidomide-refractory patients can benefit from replacing lenalidomide with pomalidomide in the treatment combination. In the OPTIMISMM study, RRMM patients with 1–3 prior lines of therapy were treated with pomalidomide, bortezomib, and dexamethasone (PVd) or Vd. In total, 71% of the PVd group and 69% of the Vd group were lenalidomide-refractory. Despite this, the median PFS for PVd was 11.2 months compared to 7.1 months for the Vd alone group, indicating significant benefits of alternative IMiD in lenalidomide-refractory patients.21
Additionally, PI- and lenalidomide-refractoriness can be overcome by using a novel PI in combination with a monoclonal antibody and lenalidomide. In the MMY1001 Phase Ib study, 82 RRMM patients were treated with daratumumab, carfilzomib (70 mg/m2 weekly), and dexamethasone, and 74% of patients achieved 12-month PFS, with 84% overall response rate (ORR). In a subpopulation of lenalidomide-refractory patients, 65% of patients were progression-free at 12 months and the ORR was 79%.24 Furthermore, the median PFS of lenalidomide-refractory patients reached 25.7 months.24
Moreover, PI- and lenalidomide-refractoriness can be overcome by combining monoclonal antibodies with pomalidomide and dexamethasone. In the ELOQUENT-3 study, patients with RRMM refractory to lenalidomide and a PI were randomly assigned to receive elotuzumab, a humanised monoclonal antibody targeting SLAMF7, plus pomalidomide and dexamethasone (EPd) or pomalidomide and dexamethasone alone (Pd). Median PFS was more than twice as long with EPd (10.3 months) versus Pd (4.7 months). Furthermore, ORR was significantly higher in the EPd group (53%) versus the Pd group (26%).22
As the population of refractory patients increases, treatment resistance is becoming a more important issue in clinical practice. Furthermore, the nature of drug resistance is evolving and diversifying because of changes in treatment standards. Concepts and strategies for tackling treatment resistance represent significant unmet needs. PI remain the foundation of MM treatment, with 2nd generation PI improving response rates, PFS, and OS. With the emerging use of lenalidomide in frontline treatment, and the resulting refractoriness, lenalidomide-sparing options are crucial when the disease inevitably relapses.
A Delicate Balance: Tailoring Treatment for Elderly Patients
Professor Xavier Leleu
As novel treatment options result in improved patient outcomes, the average age of MM patients is increasing reflecting the global trend of ageing populations. There will be an estimated 77% increase in the number of patients >65 years diagnosed with MM by 2030.25
Survival of elderly MM patients >80 years has not improved in the past 20 years.26 Furthermore, very elderly patients (≥85 years) have a significantly higher early mortality rate highlighting an emerging population with unmet need.27 Elderly myeloma patients are a heterogenous population with patient-specific challenges including old age itself, frailty, and co-morbidities, as well as cognitive, emotional, and social concerns of the patient’s life. Management of elderly patients must also consider the global health status.28 These considerations are compounded by the myeloma-specific challenges including cytogenic risk, treatment tolerability, poor performance status, and increased risk of adverse events. Furthermore, there is limited evidence from on-going and completed studies to support treatment algorithms in very elderly MM patients.
In a subgroup analysis of the ASPIRE study, patients treated with KRd or Rd were split into two age groups (<70 years or ≥70 years). In the <70 years old group, patients treated with KRd and Rd achieved a median PFS of 28.6 months and 17.6 months, respectively. Patients ≥70 years old achieved similar median PFS when treated with KRd (23.8 months) and Rd (16.0 months).29 Thus, the benefit of adding carfilzomib to Rd was conferred regardless of age. Furthermore, in a frailty subgroup analysis of the ASPIRE study, KRd improved PFS and OS outcomes versus Rd across frailty subgroups.30
The Phase III TOURMALINE-MM1 study examined the efficacy of ixazomib, an orally administered PI, with Rd (IRd) versus Rd alone. In the overall patient population (N=722), median PFS was significantly longer in the IRd group than in the Rd group (20.6 versus 14.7 months). In an age subgroup analysis, younger patients (≤65 years old) showed similar survival rates in the two treatment arms, with IRd patients achieving a median PFS of 20.6 months versus 14.1 months in the Rd group. Elderly patients (≥75 years old) achieved similar PFS rates to the total population, and median PFS was significantly longer in the IRd group compared to the control group (18.5 months versus 13.1 months).31
Subgroup analysis of the ENDEAVOR study suggested Kd treatment may confer survival rates in elderly patients comparable with younger patients. Median PFS in the youngest subgroup of patients (<65 years old) was not estimable. However, median PFS in the patients aged 65–74 years old and ≥75 years old was similar (15.6 and 18.7 months, respectively).32 OS in the age subgroup analysis was similar for patients aged <65 years old and patients aged 65–74 years (47.8 and 49.0 months, respectively), with patients aged ≥75 years achieving a lower OS rate (36.1 months).33 Furthermore, in a frailty subgroup analysis, Kd with carfilzomib at 56 mg/m2 improved PFS and OS outcomes versus Rd, across frailty subgroups.30
A subgroup analysis of patients <75 years old and ≥75 years old in the ARROW study indicated that once-weekly Kd treatment and twice-weekly Kd treatment result in similar survival rates in both age populations. Median PFS for once-weekly Kd patients <75 years old was 11.1 months compared to 12.2 months in the ≥75 years old patients. Similarly, median PFS for twice-weekly Kd patients was 7.4 months in the younger patient population versus 9.5 months in the elder population.34
Because there is limited evidence for the treatment of elderly patients, treatment should be adapted based on the patient profile. If the patient is fit, full dose therapy can be applied, including ASCT, triplet, or doublet regimens with a treatment goal of deep remission. If the patient is of intermediate frailty, the treatment goal should be a balance of safety and efficacy. Therapy options should be reduced to doublet regimens or reduced-dose triplet regimens. However, if the elderly patient is frail, safety and tolerability of treatment should be the highest priority with reduced-dose doublet therapy regimens being the main option.35
Key Phase III trials have shown that elderly patients derive clinical benefit from novel drug combinations, such as KRd, Kd, DRd, DVd, and IRd. To date, there are no treatment regimens indicated specifically for the elderly population, therefore treatments should be chosen based on safety signatures. All drugs can be applicable to elderly fit patients and treatment can be the same as for non-elderly patients. Furthermore, all drugs can be considered for elderly frail patients, with a focus on doublet regimens instead of triplets. Elderly myeloma patients should be carefully monitored for the emergence of treatment side effects and managed accordingly.
Big Changes May Arise: Evolution of Immunotherapies
Professor Hermann Einsele
In recent years, targeted immunotherapy has become a major focus for treatment of MM, aiming to personalise therapy, improve outcomes, and bypass the issues of drug resistance. Novel immunotherapies targeting T cell receptor activity, including bispecific T cell engagers (BiTE) and chimeric antigen receptor (CAR) T cells, are currently in clinical development for the treatment of MM.36,37 Both BiTE and CAR T therapy trigger tumour cell lysis via T cell-mediated cytotoxicity. BiTE molecules redirect cytotoxic T cells toward myeloma cells, and CAR T therapy relies on generating large numbers of tumour-reactive T cells that are capable of initiating myeloma cell apoptosis.38 Despite promising efficacy of T cell redirection strategies, they are associated with cytokine release syndrome (CRS) and neurotoxicity which need to be carefully managed.
BiTE molecules are created by linking the targeting regions of two individual antibodies with a peptide linker in order to increase tumour-engaging T cell activity. The antibodies are designed to target specific receptors on the tumour cell and endogenous T cells, allowing the T cell to recognise the tumour cell and initiate apoptosis. Early data from the first in human, Phase I study of AMG 420, an anti-BCMA BiTE, showed encouraging results in heavily pretreated RRMM patients. At the maximum tolerated dose of 400 µg per day, 7 out of 10 patients achieved a partial response (PR) or better (5 MRD-negative, 1 very good partial response, and 1 PR), with a median response duration of 9 months, ranging from 5.8–13.6 months. At doses <800 µg per day, no major toxicities of CRS and polyneuropathy were observed, and no anti-AMG 420 antibodies were detected.39
CAR T cells are genetically engineered cells generated from the patient’s own T cells. Collected cells are transduced with CAR DNA that incorporates into the genome and results in the expression of CAR proteins on the cell surface. CAR T cells are then delivered to the patient to attack the tumour cells. Early analysis of an on-going Phase I study of LCAR-B38M CAR T cells, which targeted BCMA proteins on myeloma cells, showed promising results. Of the 57 patients evaluable at the data cutoff, the ORR was 88%, with 68% patients achieving CR, 5% achieving a very good partial response, and 14% achieving a PR. Overall, 64% of patients achieved MRD-negativity. CRS occurred in 90% of patients, with 4 patients experiencing Grade ≥3 cases.40,41
Moreover, data from the on-going bb2121 Phase I study also showed CAR T cells targeting BCMA as potentially clinically efficacious. The ORR was 85%, including 15 patients (45%) with CR, and the median PFS was 11.8 months. The median PFS was significantly longer (17.7 months) in 16 patients who were MRD-negative. In this study, 63% of patients had CRS, which was mostly Grade 1 or 2. CAR T cell expansion was associated with responses, and their numbers persisted up to 1 year after the infusion.42 CRS can occur up to 16 days after CAR T cell infusion and persist for several days to weeks, contrasting with the rapid CRS response observed following BiTE infusion (within 72 hours of treatment).43 The safety profile of CAR T cells may be improved by modulating the activity of CAR T post-infusion. Early preclinical animal model work suggests that the CRS response may be mitigated by using tyrosine kinase inhibitors. Moreover, this CAR T cell inhibition is fully reversible to reinstate their function when required.
Novel immunotherapies are highly active in patients with heavily pretreated MM, inducing MRD-negative CR in most patients. However, longer follow-up observations are needed to assess whether long-term PFS can be achieved at least in a subgroup of patients. Target antigen loss will be a major problem for all the T cell redirection strategies, thus simultaneously targeting additional MM pathogenic pathways may be necessary to mitigate this issue, especially in the pretreated MM patient. Moving T cell redirection strategies to earlier lines of therapy is likely to increase the efficacy, and additionally improve patient outcomes.







