Meeting Summary
The limitations of currently available therapies for immune thrombocytopenia (ITP) mean that long-term responses are difficult to maintain, and patients face a substantial quality of life (QoL) burden imposed by both the disease and its treatment. During this Sanofi industry-supported symposium, leading experts in haematology discussed the significant unmet needs that exist in ITP, and highlighted the importance of new treatment approaches on the horizon with the potential to deliver improved clinical outcomes for patients. Waleed Ghanima, Consultant Hematologist at Østfold Hospital and Professor at the Institute of Clinical Medicine, University of Oslo, Norway, reviewed the key clinical considerations when choosing treatment for ITP and risk factors associated with established therapies. Cindy Neunert, Professor in the Department of Pediatrics and Section Head of Pediatric Hematology at Columbia University Irving Medical Center, Manhattan, New York, USA, explored patient preferences and goals of therapy in ITP, moving beyond platelet count to address important concerns such as fatigue and cognition. David J. Kuter, Chief of Hematology at Massachusetts General Hospital and Professor of Medicine at Harvard Medical School, Boston, USA, highlighted the promise of emerging therapies on the horizon for ITP that address multiple mechanisms of disease pathology and may help to fill existing treatment gaps.
What are the Key Clinical Concerns When Choosing Treatment?
Waleed Ghanima
Treatment goals in ITP are dynamic and change with disease duration and severity.1-3 The main objective is prevention or termination of bleeding, but as Ghanima explained, QoL and avoidance of adverse events (AE) become increasingly important in chronic disease. Data from the I-WISh patient survey of 1,500 patients with ITP from 13 countries indicate that healthy blood counts (64%), prevention of worsening episodes (44%), increased energy levels (41%), and improved QoL (38%) rank among the top treatment priorities for patients.4,5
Corticosteroids are the current first-line management for ITP, but most patients will require subsequent therapy. Second-line treatment options include immunomodulation with the anti-CD20 monoclonal antibody (mAb) rituximab or spleen-associated tyrosine kinase (Syk) inhibitors, notably fostamatinib; splenectomy to remove the site of platelet destruction; or increasing platelet production with thrombopoietin receptor agonists (TPO-RAs).1,3 However, Ghanima highlighted the limited durable response and remission rates achievable with most currently available second-line therapies as a key concern when choosing treatment. For TPO-RAs, durable response rate, which he described as a “very important endpoint” when assessing efficacy, ranges from around 34% with avatrombopag to around 60% with romiplostim and eltrombopag.6-8 For splenectomy and rituximab, the durable response rate is around 50%, while fostamatinib falls substantially shorter at just 18%.2,9,10 In terms of sustained responses off-treatment, splenectomy is the strategy that supplies the highest remission rate: 69% at 1 year compared to 24% for rituximab.2 Accumulated data on TPO-RAs indicate that only around 25–32% of patients are able to maintain stable platelet counts 6–12 months after therapy discontinuation.11-13
Side effects constitute another key consideration when selecting ITP therapy, most notably infections and the risk of thromboembolism. Registry studies have confirmed that infection risk is heightened in the ITP patient population.14,15 Looking at the impact of available ITP therapies and treatment strategies on infection risk, splenectomised patients were shown to have an increased risk of infection versus non-splenectomised patients during the first 90 days (adjusted risk rate: 2.6), which persisted for over 1 year.16 In a French non-splenectomised cohort study, the incidence rate of serious infections was found to be 6.3 per 100 patient-years (PY) and every patient had at least one non-serious infection per year (incidence rate: 100.5/100 PY).17 This study also showed that exposure to rituximab and corticosteroids was associated with an increased risk of both serious infections (2.60 and 3.83, respectively) and non-serious infections (1.49 and 2.46, respectively). With corticosteroids, infection risk was present from averaged daily doses of 5 mg prednisone equivalent, which Ghanima described as “interesting” given these would be considered relatively low corticosteroid doses in clinical practice.18 With its prolonged B cell-depleting effects, standard-dose rituximab is also associated with an increased infection risk ratio (RR), estimated at 1.35 in a recent meta-analysis.19,20 Ghanima stressed that, despite the ability to treat infections with antibiotics, they remain one of the key causes of reduced survival in patients with ITP, and hence an important concern with available therapies.21
Thromboembolism represents another important risk with currently available ITP therapies. Large observational studies conducted before the advent of TPO-RAs indicated a significant RR of arterial and venous thromboembolism of 1.5 and 1.9, respectively, among patients with ITP.22 Thromboembolic events were observed in most of the TPO-RA trials and a recent meta-analysis estimated an OR of 1.76 for thromboembolism risk in patients treated with TPO-RA, albeit not significant (p=0.18).23 This risk was confirmed by a Mendelian randomisation analysis that suggested an increased risk of thromboembolic events, particularly venous thromboembolism, deep vein thrombosis, and stroke, in patients receiving TPO-RAs.24 A further single-centre study of 220 patients identified a venous thromboembolism rate of over 2.05/100 PY in patients with ITP, with TPO-RA use found to be a predictive factor on multivariable Cox regression analysis (hazard ratio: 2.96).25 Collectively, these results suggest an increased risk of thromboembolism in the TPO-RA-treated ITP population, Ghanmia surmised, although more studies are needed to confirm this finding.
Finally, clinicians must consider gastrointestinal toxicity, which is a common AE observed with Syk inhibitors, with diarrhoea occurring in around one-third of patients treated with fostamatinib in the Fostamatinib for Immune Thrombocytopenia (FIT) study.10,26 Although usually mild-to-moderate, Ghanima noted that this diarrhoea can sometimes be severe and require medication discontinuation. Potential hepatotoxicity also demands that patients’ liver enzymes be followed up during treatment, he added.
Ultimately, Ghanima stressed that patient satisfaction with treatment remains the most important metric. Results from the I-WISh study indicate that this is lowest with corticosteroids.27 Over half of patients on corticosteroids, and around one-third of those on anti-CD20 mAbs and TPO-RAs, said they did not want to take their current medications for the foreseeable future. The majority of patients with ITP also expressed worries about both the short- and long-term side effects of their current treatments (Figure 1).
In summary, the main efficacy limitations of current therapies for ITP lie in the limited rates of durable responses and remissions. Key safety concerns include the increased risk of infection associated with immunosuppressants and the increased risk of thromboembolism associated with TPO-RAs. One of the main problems facing clinicians is that neither response nor side effects with existing treatments can be predicted. There is therefore still a need for therapies with both improved efficacy and safety profiles, Ghanima concluded.
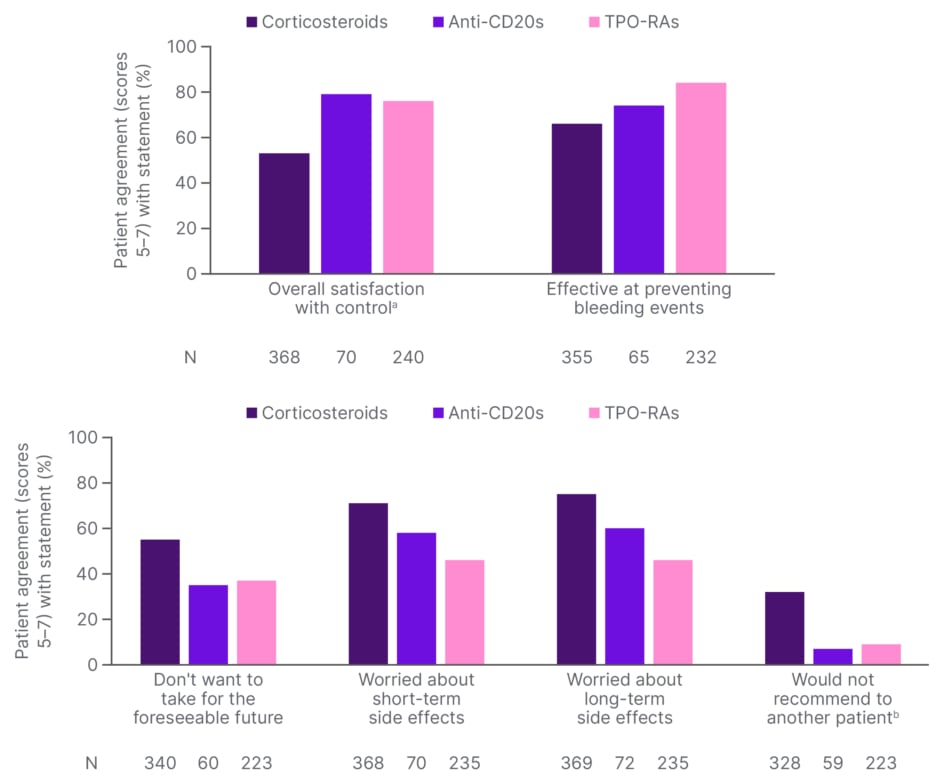
Figure 1: Patient satisfaction with current treatments (I-WISh). 27
aPlatelet count control
bDisagreement scores with “would recommend to another patient” statement (score ≤3) have been banded and displayed as an agreement with “would not recommend to another patient” to allow a direct comparison with the other statements.
CD: cluster of differentiation; I-WISh: ITP World Impact Survey; TPO-RA: thrombopoietin receptor agonist.
How to Navigate Patient Concerns with Clinical Outcomes?
Cindy Neunert
American Society of Hematology (ASH) treatment guidelines and International Consensus Recommendations, both published in 2019, are centred around objective evidence of benefit in ITP, primarily platelet count outcomes balanced against reported AEs.2,3 It can therefore prove challenging to translate these guidelines into clinical care that truly reflects the subjective patient experience, Neunert explained.
There is also evidence to show that existing ITP guidelines are not a key driver for clinical decision-making. In the Pediatric ITP Consortium of North America study, where patients were transitioning from first- to second-line therapy, 53% of physicians indicated parental or patient preference as the top reason for selecting a specific treatment.28 The possibility of remission also ranked highly, but only 9% of doctors relied on published guidelines to direct their treatment choice.28 Neunert suggested that the reason for this disconnect between evidence and clinical decision-making is that platelet count is a historical surrogate endpoint that does not accurately reflect the entire disease burden experienced by patients.3 Platelet count is just a laboratory number, she stressed, and does not fully capture the important clinical aspects of ITP.
Physicians therefore need to move beyond platelet count and consider the therapy-related disease impact, such as AEs and direct/indirect costs, as well as the patient experience in terms of bleeding severity, health-related QoL (HRQoL), fatigue, and cognition, alongside the priorities of patients and their families.3,4,29 There is also a need to expand the incorporation of HRQoL measures into clinical studies. A recent systematic review looking at 168 published ITP trials between 2010–2019 found that only a handful included HRQoL as an outcome.30
Survey data from the ITP World Impact Survey (iWISh) study highlight the significant impact of the ITP disease burden on patients’ daily living. More than 40% of patients reported some impact of ITP on energy levels, 34% on their capacity to exercise, and over 20% on tasks, hobbies, work and/or social life.5 Those patients with a very high symptom burden were also more likely to reduce their hours at work or consider leaving their jobs. Patients consistently ranked fatigue as a key ITP symptom in terms of both frequency and severity.5 At diagnosis, 58% of patients said that fatigue was their most commonly occurring symptom and 73% ranked it as the most severe. Fatigue was also the top symptom that patients wanted to resolve (46%).5 Neunert contrasted this with physicians who typically place bleeding at the top of the list, with fatigue much lower down, and emphasised the importance of aligning priorities in patient care. Cognition is another key area that can be impacted by ITP, as shown by data from Phase I/II trial of rilzabrutinib that evaluated four key cognitive domains in 60 patients.31 Almost 70% of patients with baseline Cogstate Brief Battery (CBB) assessments showed combined psychomotor and attention impairments, while over 40% showed impairments in the composite score of visual learning and working memory.31 This illustrates that almost half of patients with ITP were experiencing some degree of cognitive impairment in at least one domain, Neunert pointed out.
Although guidelines provide an important evidence-based foundation for clinical care, we need to move beyond them, Neunert urged, towards a new model of shared decision-making in ITP. Important steps to achieving this include dissemination of guidelines to patients, more frequent reporting of patient-related outcomes such as HRQoL and fatigue, the capture of real-world data on ITP treatment outside the controlled setting of clinical trials, and an increased focus on the patient experience.32
Shared decision-making itself represents a process by which clinicians and patients come together in a deliberate conversation to align their respective priorities, which can be facilitated by decision-aids. Physicians input evidence and clinical experience, while patients share their values and contextual experiences of ITP. From this emerges ‘evidence-based patient preference’ and a final decision around management. Ultimately, the aim is to move towards individualised ITP management, Neunert stressed, which, in the ITP space, involves setting goals around benefits, balanced against risks, to achieve the desired outcome of ‘a healthy patient’ (Figure 2).
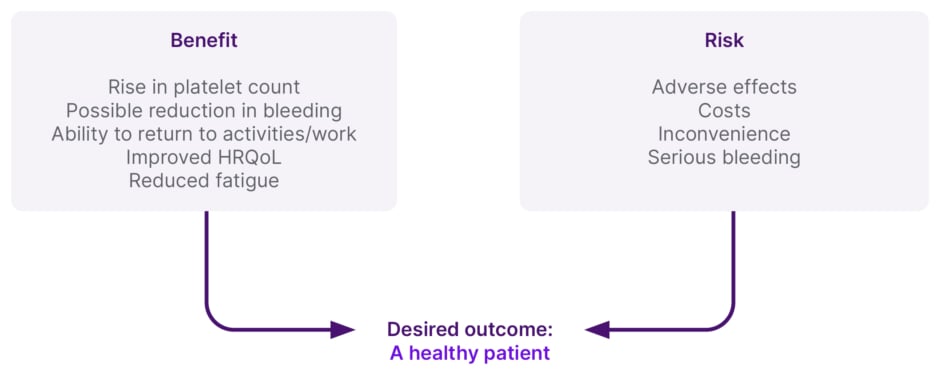
Figure 2: Individualised management of immune thrombocytopenia.
Patient preference is a large driver of decision-making in immune thrombocytopenia, and it is important to understand the true clinical burden of the disease on patients. Engagement in shared decision-making is essential as this increases patient understanding of treatment options, improves patient-related outcomes and the achievement of key goals, and helps to guide treatment decisions.
Adapted with permission from C. Neunart.
HRQoL: health-related QoL.
Can Emerging Treatments Help Fill the Gaps?
David J. Kuter
With a plethora of new treatment approaches on the horizon for ITP, Kuter gave a pathophysiological overview of the disorder and outlined the mode of action (MOA) of novel therapies in clinical development. According to the new model of ITP, underlying disease pathophysiology results from both a high rate of platelet destruction and an inappropriately low rate of platelet production. Hence the disease can be treated in two key ways, either decreasing destruction or boosting production of platelets.
Kuter focused first on ITP as a disorder of impaired platelet production.33,34 Treatments that target this pathway include corticosteroids, recombinant human TPO, TPO-RAs, and low-level laser light.35,36 Hetrombopag, the newest agent in the TPO-RA class has shown to be 10 times more potent than eltrombopag in stimulating megakaryocyte growth.37 In the largest ITP study to date, hetrombopag led to a significant rise in platelet counts at both 2.5 and 5 mg doses, which was maintained for the 10-week, double-blind treatment period.38
ITP is also a disorder of increased platelet destruction.39,40 Therapeutic approaches that aim to reduce the production or survival of anti-platelet antibodies are numerous and include approved therapies like corticosteroids and rituximab, as well as investigational approaches such as anti-CD38 mAbs, immunoproteasome inhibitors, neonatal Fc receptor (FcRn) inhibitors, B-cell activating factor (BAFF) receptor inhibitors, and IgG proteases.35,41,42 Bruton’s tyrosine kinase (BTK) inhibitors and Syk inhibitors might also act by reducing the production of autoantibodies.35
The FcRN receptor binds two ligands (IgG and albumin) avidly at low pH and at non-overlapping sites and prevents their degradation.43 Inhibition of the IgG binding site at the FcRN receptor leads to reduced half-life of normal and pathological IgG thereby reducing their levels in the circulation.35 Efgartigimod (ARGX-113) is an FcRN inhibitor, approved by the FDA as a treatment for myasthenia gravis, which was developed with ABDEG technology to create a ‘sticky’ IgG with increased affinity for FcRN and a slow off-rate at pH 7.44,45 In the ADVANCE trial, intravenous efgartigimod led to a drop in IgG levels of over 60%, which was maintained for the 24 weeks of study.46,47 The primary endpoint of sustained platelet count response was achieved in 21.8% of patients versus 5% on placebo.46,47 Kuter described these responses as “modest,” likely because patients had already exhausted multiple prior therapies and were decades into their ITP journey. However, a subsequent study of the subcutaneous formulation of efgartigimod failed to meet the primary or any of the secondary endpoints.48
BAFF is elevated in many autoimmune diseases, and the BAFF receptor inhibitor VAY-736 is under evaluation in multiple studies for early and chronic ITP.49,50 Its MOA involves binding to and inhibiting the BAFF receptor, thereby leading to reduced B cell function and B cell depletion.51,52
Potent IgG cleaving and degrading enzymes produced naturally by some bacteria also hold therapeutic interest in ITP.53 A recombinant form of one of these enzymes, imlifidase, successfully eliminated donor-specific antibodies in a cohort of kidney transplant recipients, thereby permitting HLA-incompatible organ donation. Kuter described imlifidase as an “interesting” molecule but cautioned that its high immunogenicity allows for one-time use only.53 Newer, less antigenic, forms are being developed.
Finally, Kuter turned to inhibitors of macrophage function as treatment approaches to ITP.35 Approved therapies that exploit this MOA include corticosteroids, vincristine/vinblastine, and intravenous immunoglobulin, while agents under clinical development include novel Syk kinase inhibitors and BTK inhibitors.35,54 Sovleplenib, a more specific and potent Syk kinase inhibitor than fostamatinib, demonstrated a sustained, durable platelet response in a recently published Phase III randomised, controlled trial.55 In this study, sovleplenib achieved a durable response rate of 48.4% (versus 0% on placebo), with a tolerable safety profile and significant improvements in patients’ QoL domains for physical function and energy/fatigue.55
The resolution of autoimmune cytopenias with ibrutinib in patients with CLL has generated “great interest” in developing a new BTK inhibitor for ITP, Kuter explained.56 Rilzabrutinib is a selective and reversible BTK inhibitor with a dual MoA in ITP (Figure 3). It inhibits macrophage-mediated platelet destruction by preventing FcyR-mediated phagocytosis and also inhibits B cell activation, thereby potentially reducing the production of pathogenic autoantibodies.60,61 In contrast to ibrutinib, rilzabrutinib is targeted and has no anti-platelet effect, Kuter noted. In a Phase I/II trial in previously-treated ITP, 40% of patients achieved a response to rilzabrutinib, meeting the study’s primary endpoint.62-64 Importantly, Kuter described this response as “well maintained,” with platelet counts sustained above 30,000 x109/L for a median of 95% of weeks in rilzabrutinib responders. The side effect profile was modest with no reported Grade 3/4 events of diarrhoea, nausea, or fatigue. A pivotal Phase III study of rilzabrutinib (LUNA) has now been completed, in which all the major endpoints were met, confirmed Kuter, with full data to be presented later this year.65 In the LUNA 3 trial, rilzabrutinib demonstrated a significant and durable platelet response versus placebo in patients with ITP refractory to prior therapy.66
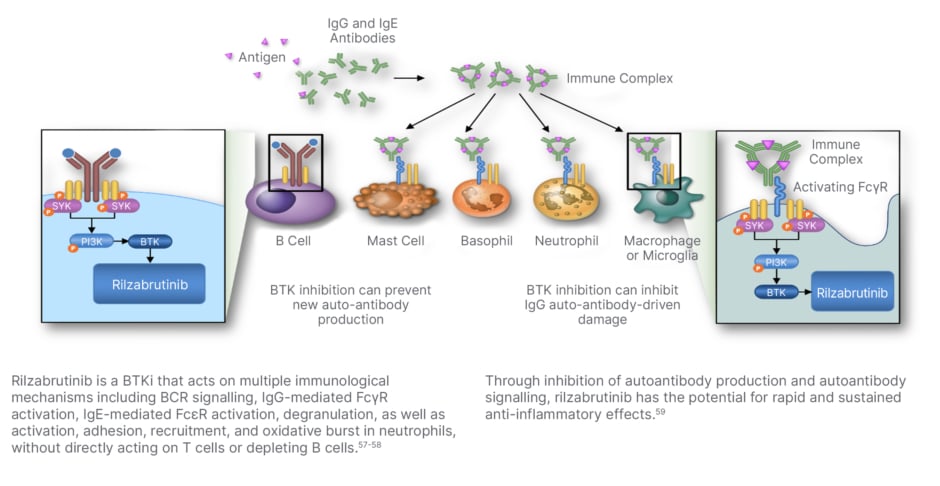
Figure 3: Inhibition of Bruton’s tyrosine kinase by rilzabrutinib.
BCR: B-cell receptor; BTK: Bruton’s tyrosine kinase; BTKi: BTK inhibitor; FcγR: fragment crystallizable-gamma receptor; FcεR: fragment crystallizable-epsilon receptor; IgE: immunoglobulin E; IgG: immunoglobulin G; ITP: immune thrombocytopenia; MoA: mechanism of action; PI3K: phosphatidylinositol 3-kinase; Syk: spleen tyrosine kinase.
In summary, ITP pathophysiology is complex and understanding it can help to guide the development of new treatments. Existing treatment options such as corticosteroids and TPO-RAs act to increase platelet production. Many novel therapies are also on the horizon targeting the increased platelet destruction associated with ITP. This includes agents aimed at reducing antiplatelet antibodies, blocking complement, or inhibiting macrophage function. When evaluating study data on these ‘exciting’ new treatment options for ITP, Kuter reiterated the importance of considering, not only platelet outcomes, but QoL and other key treatment goals.
Q&A
All panel
The main symposium presentations were followed by a question-and-answer session in which all panel members participated.
When asked whether any of the current second-line ITP agents may be used first-line in the future, Ghanima noted that several trials are ongoing, testing upfront combinations of dexamethasone and RAs, which, if successful in prolonging the durability of response and remission rate, might change clinical practice.
In answer to a question on mechanisms of TPO-RA resistance, experts flagged the importance of considering adherence as a potential cause of loss of response. Kuter also alluded to data suggesting that patients with a high endogenous TPO level are less responsive to TPO-RAs.67
Asked about the potential to achieve durable responses with low doses of corticosteroids (e.g., 5 or 10 mg) over a long period, the panel acknowledged that some steroid-sensitive patients can be well-maintained on this approach, even though guidelines endorse shorter courses of steroids with taper.
Experts were questioned about potential clinical scenarios where risks associated with SoC would prompt use of a non-guideline-directed therapy. Kuter highlighted specific patient factors such as age, comorbidities, and bleeding risk, as well as secondary ITP. Ghanima added that choice between TPO-RA, fostamatinib, and rituximab should be personalised depending on the individual risk of thrombosis and infection.
Neunert was asked about the association between fatigue in ITP and low ferritin levels. She stressed the importance of ruling out potential organic causes (such as iron or thyroid deficiency) and lifestyle concerns, but conceded that the full pathology of fatigue in ITP, and hence the optimal therapeutic approach, is not yet understood.
Asked about myelofibrosis risk with TPO-RAs, Ghanima conceded that studies have shown a slight increase in myelofibrosis in the bone marrow in the majority of patients, albeit not clinically significant. Grade 2 or 3 reticuline and collagen is occasionally seen on bone marrow biopsy, he noted, but is usually reversible once TPO-RAs are discontinued.
On the topic of combination therapy in ITP, experts highlighted the potential for synergy between agents from different drug classes, leading to augmented clinical effects that may be particularly beneficial in refractory patients.
The panel acknowledged that current ITP guidelines are biased towards resource-rich regions and that, despite the host of new agents under clinical evaluation, older therapies may still have a role to play in resource-poor settings.
Finally, experts were asked about the potential for novel drugs under development for ITP to finally cure the disease. Kuter confirmed that, in addition to improving platelets and QoL, attaining treatment-free remission remains the ultimate ‘aspiration’ in ITP.
MAT-GLB-2405640-1.0 – 08/2024






