Abstract
This review will cover the epidemiology, risk factors, pathogenesis, and pathology of hepatocellular carcinoma (HCC). HCC is the fifth most commonly diagnosed cancer in males and second most frequent cancer-related cause of mortality worldwide. In females, it is the seventh most frequently diagnosed malignancy and sixth leading cause of death. The incidence of HCC is higher among males in less developed countries and reaches a peak around the age of 70 years. The rates of liver cancer are twice as high in males compared to females.1,2 Various risk factors, including environmental, infectious, nutritional, and metabolic, are associated with HCC; among them viral infection has been linked to being the highest risk factor for developing HCC.
HCC is a highly vascular tumour and its pathogenesis consists of increasing angiogenesis by overexpression of various growth factors. Another cause of HCC development is thought to be mutations in different signalling pathways that lead to proliferation of the tumour cells.
EPIDEMIOLOGY
Hepatocellular carcinoma (HCC) is the most common primary liver cancer; it accounts for 70–85% of the total incidences of liver cancer and is the third leading cause of cancer mortality worldwide.1 It accounts for 9.2% of the new cancer diagnoses worldwide with >748,000 new cases per year.3 It usually appears after the age of 40 years and reaches a peak around 70 years of age,4 with an overall 5-year survival of <12% in the USA.5 HCC distributions differ by race, ethnicity, geography, and sex. For instance, Hispanics and AfricanAmericans in the USA have higher rates of HCC than non-Hispanics but lower rates than Asians.6 Furthermore, HCC has a high incidence in subSaharan Africa and Southeast Asia.4 The incidence in Caucasians in the UK and USA is lower,7 and a probable cause for this is the different aetiologies of HCC. Table 1 demonstrates the incidence of HCC in various countries worldwide.5
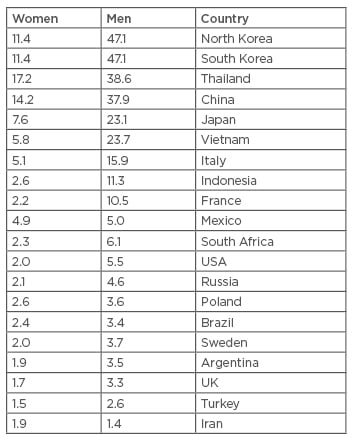
Table 1: Age-standardised incidence rates of hepatocellular carcinoma per 100,000 populations at risk, in different regions of world.5
RISK FACTORS
Non-Modifiable Causes
Non-modifiable causes also include factors related to age, sex, and ethnicity. It has been shown that black, white, and Hispanic men >50 years of age have an increased risk of developing HCC. In ages ranging between 35 and 49 years there has been a decrease in the incidence of HCC.8 Males show a higher risk for HCC than females due to differences in exposure to risk factors such as hepatitis B virus (HBV) and hepatitis C virus (HCV).9 Family history of liver cancer in first-degree relatives was associated with a significantly elevated risk of liver cancer.10
Modifiable Causes
Hepatitis B virus
HBV infection correlates to >50% of HCC cases. In HBV endemic areas, HBV has the potential to become chronic in >90% of the individuals; the infection is transmitted by perinatal or vertical ways. In developed countries, there is low potential of chronic infection and >90% of cases resolve spontaneously, with the transmission usually associated with sexual and parenteral routes.5
Geographical region
HCV prevalence in patients with HCC can vary between 20% and 90%, depending on the geographical region.11 The USA, Europe, and Japan have the highest incidence of HCV-infected patients. Compared to negative HCV patients, chronic HCV patients have a 17-times higher risk of developing HCC.12
Non-alcoholic fatty liver disease
In a population-based study, a statistically significant association was shown between metabolic syndrome and risk of HCC, making non-alcoholic steatohepatitis (NASH) the contributor. In cohort based studies including patients with non-alcoholic fatty liver disease (NAFLD), there was no increased incidence of HCC.5 NASH, which is a subset of NAFLD, showed an increasing incidence of liver transplantation due to HCC, which reached ≤6% of the overall number of liver transplantations undergone in 2012 in the USA, while in 2002 this figure was 0%.13
Excessive alcohol consumption, defined as a daily intake of alcohol for 10–12 years with doses in excess of 40–80 g/day for males and 20–40 g/day for females, may lead to alcoholic liver disease, which in turn may lead to cirrhosis and finally to HCC. Out of long-term heavy alcoholic drinkers it is reported that only 10–35% will develop alcoholic hepatitis and only 8–20% will develop cirrhosis.14
Aflatoxins
Aflatoxins are metabolites derived from the fungi Aspergillus flavus and Aspergillus parasiticus. They can be found in maize, ground nuts, and rice in tropical and subtropical countries. The mycotoxin optimally grows at temperatures between 25oC and 32oC in a moist environment. Aflatoxin B1 is the most potent experimental hepatocarcinogen known and is considered to be a cause of HCC development.15
Haemochromatosis
Haemochromatosis is a genetic disorder in which iron deposits in the tissues can lead to liver damage. Homozygosity of the C282Y mutation on the HFE gene is associated with low secretion of hepcidin, which is the regulator of iron metabolism in the body. When iron overloads over time in the body, the hepatocytes become damaged and dysfunctional, which leads to cirrhosis and HCC. HCC develops in 8–10% of patients with hereditary haemochromatosis. HCC is the major cause of mortality in individuals with hereditary haemochromatosis, where it reaches ≤45%.16
Wilson’s disease
Wilson’s disease (WD) is an autosomal recessive disorder in which copper accumulates in the tissues. Its manifestation includes neurological, psychiatric, and liver disorders. Whether HCC develops as a result of WD is controversial. Animal studies show that the use of chelating agents decreases the risk of HCC in these patients, while in clinical and experimental trials it has been shown that tumourigenesis in WD patients is multifactorial and that chronic liver injury (rather than copper accumulation) leads to HCC.17
Diabetes mellitus
Diabetes mellitus (DM) was significantly associated with increased risk of HCC as shown by a meta-analysis.18 Among diabetics using metformin, HCC incidence was lower, but in diabetics using sulfonylurea a higher risk was indicated.19 The cause for the increased risk of HCC in Type 2 DM is not fully understood and was attributed to the development of NAFLD, which would cause chronic liver injury and later the development of HCC.20
Contraceptives
Oestrogen-progestogen contraceptives are classified by the International Agency for Research on Cancer (IARC) as a cause of HCC.21 However HCC risk is not associated with the use of oral contraceptives.22
Occupational exposures
Vinyl chloride has been classified by the IARC as a cause of HCC.21 A follow-up study that included 1,600 male Italian autoclave workers had an almost 10-fold higher risk of HCC due to exposure to vinyl chloride gas compared to individuals who were not exposed.23
PROTECTIVE FACTORS
Several studies have demonstrated the protective role of metformin. This medicine is considered as an anti-cancer and anti-oxidant agent for solid tumours (colon, prostate), as metformin decreases insulin resistance. It promotes a decrease in plasma insulin (which is known as a growth promoting factor with direct mitogenic effects) and a decrease in glucose levels in patients with Type 2 DM, which is a known risk factor of HCC. The ability of metformin to decrease the plasma insulin is the reason for its proposed anti-oncogenic mechanism.24
Several meta-analyses suggest that coffee, statins, and vegetables have an inverse relationship to the development of HCC.25-27 Coffee has a protective effect in the prevention of HCC. A possible explanation could be related to the inclusion of paraxanthine. This metabolite inhibits the connective tissue growth factor found in the hepatic parenchyma and thus protects the liver from HCC. It has been shown that the intake of two cups per day reduces the risk of HCC development by 43%.25 Another meta-analysis also indicates that one cup of coffee per day decreases the risk of HCC and there is an inverse relationship between the amount of cups consumed and the risk of HCC.28 Statins are medications that inhibit 3-hydroxy-3methylglutaryl-coenzyme A (HMG-CoA) reductase, which leads to the inhibition of myelocytomatosis oncogene (Myc) phosphorylation by having an anti-oxidant effect. These mechanisms lead to tumour suppression and decrease the risk of HCC.26 Statins are also known to be used in the treatment of metabolic syndrome, also recognised as a risk factor that can lead to NASH. Particular interest is focussed on the impact of statins on the development and progression of neoplasms. Furthermore, statins have been shown to cause apoptosis in cultured cancer cells through this same method, specifically by inhibition of geranylgeranyl pyrophosphate production. Another mechanism proposed is that statins may prevent HCC through an indirect effect by prevention of HCV replication.29
Vegetables contain phytochemicals, such as isothiocyanates, glucosinolates, indole-3-carbinol, and flavonoids, that have anti-tumour effects, although the mechanism of protection is not completely clear. It was also reported that Vitamin E has been found to decrease the risk of HCC.27 Consumption of fish has been shown to decrease the risk of HCC development due to their rich source of omega-3 polyunsaturated fatty acids, such as eicosapentaenoic acid, docosapentaenoic acid, and docosahexaenoic acid, which have anti-inflammatory properties. They lead to a decrease in interleukin (IL)-1 and IL-6 pro-inflammatory activity, which is known to contribute to HCC development.29-31
PATHOGENESIS
HCC pathogenesis is multifactorial, as different factors contribute directly or indirectly to hepatocarcinogenesis. Environmental, infectious, nutritional, and metabolic factors are all associated with development of HCC.32 Several genetic pathways have been considered as a cause of HCC, including post-injury regeneration of hepatocytes, angiogenesis, and activation of signalling pathways.33 Reactive oxygen species and reactive nitrogen species have also had an effect on the regenerative, respiratory, and energetic pathways in the liver cells.34 Hepatocytes that were injured due to liver injury, such as viral hepatitis, NAFLD, or DM, have the ability to regenerate from stem cells. The occurrence of gene mutations in components of the Wnt/β-catenin signalling pathway, such as axin and β-catenin, takes place during the renewal of stem cells and results in the formation of HCC cells.33,35,36
Role of Growth Factors and Angiogenesis in Hepatocellular Carcinoma
HCC has high vascularity, which contributes to the growth of the tumour. Several growth factors are attributed to the angiogenesis, cell proliferation, and metastatic activity. Among them vascular endothelial growth factor (VEGF), platelet-derived growth factor, epidermal growth factor, fibroblast growth factor (FGF), and insulin-like growth factor can be found in tumour cells as well as their surrounding cells.33 Growth factors affect surrounding cells in both paracrine and autocrine fashion and contribute to the proliferation of the cells. Treatment for HCC can be associated with targeting these growth factors.33 Thrombosis in the portal vein is a complication that can be seen in high expression of growth factors.37
Role of Signalling Pathways and Genetics in the Development of Hepatocellular Carcinoma
Telomerase reverse transcriptase (TERT), VEGFA, FGF19, tumour protein p53, and epigenetic modifiers such as AT-rich interactive domain-containing protein 1 (ARID1) play a role in the formation of HCC.38 In addition to these genetic modifications, signalling pathways were also associated with hepatocarcinogenesis, including, the Wnt/β-catenin pathway and receptor TK-activated pathways, including the Ras/Raf/MEK/ ERK pathway and the PI3K/AKT/mTOR pathway.39
The most frequent mutation that leads to HCC is associated with TERT promoter (≤60% of cases). TERT is a part of the telomerase complex together with the telomerase RNA component. Telomerase reactivation is a key factor in malignant transformation and it occurs in >90% of HCC, due to TERT promoter mutation, TERT amplification, or HBV insertion in the TERT promoter. In 6% of low grade dysplastic nodules and in 19% of the high-grade dysplastic nodules there are TERT promoter mutations. There are dramatic increase in TERT mutations in early HCC, while in advanced and progressed HCC, TERT mutations remain stable.38
The tumour protein p53 cell cycle pathway mutation is associated with up to half of HCC patients (12–48%) and is related mainly to aflatoxin exposure. Mutations in the retinoblastoma pathway, cyclindependent kinase inhibitor 2A (CDKN2A) deletion (2–12%), and RB transcriptional corepressor 1 (RB1) mutation (3–8%) were associated with HCC.38
SWItch/sucrose nonfermentable (SWI/SNF) chromatin remodelling complexes modify chromatin structure and nucleosome position. They modify the transcription fate of the cell in an indirect way. These complexes have tumour suppression effects and cause inactivation of the ARID1A and ARID2A, members of the SWI/SNF that are seen in HCC.38
VEGFA could have a double effect in the tumourigenesis of HCC, firstly by angiogenesis and secondly by inducing overexpression of hepatocyte growth factor. High levels of VEGFA are correlated with poor survival.38 FGF19 is described in 5–14% of HCC cases, and high levels of expression are associated with poor prognosis of HCC.38
The Wnt/β-catenin pathway plays a role in intracellular signalling and cell-cell interactions. This pathway is often activated by a CTNNB1 mutation (11–37%) and by an inactivating mutation of AXIN1 (5–15%) or APC (1–2%). This leads to accumulation of β-catenin in the cytoplasm and leads to stimulation of genes associated with cell proliferation, angiogenesis, and anti-apoptotic effects that take part in malignant transformation.38,39 The Ras/Raf/MEK/ERK signalling pathway has a role in extracellular signal transduction, cell growth, and survival. The continuous activation of this pathway by tyrosine kinase ligands is significant in HCC development.39 The PI3K/AKT/mTOR pathway has a key role in cellular proliferation and cell survival. PI3K activates AKT which in turn inactivates pro-apoptotic proteins such as Bcl-2-associated death promoter (BAD) and caspase-9; this leads to the survival of cancer cells. Considering mTOR function in regulation of cell translation, an examination of HCC cases found that mTOR had been upregulated.39
Telomerase is an enzyme which protects chromosomes. With each cell cycle the telomere length reduces, and when the telomere length is too short they signal for apoptosis. If inactivation in the protective mechanism occurs, the chromosomes become unstable which in turn leads to cell proliferation.40 Telomerase activity in liver tissue of the normal population has either low or undetectable levels of this enzyme, while high levels of telomerase can be found in HCC patients.41
PATHOLOGY
Gross morphology of HCC ranges from single mass to multi-nodular masses with diffusely scattered foci. Multi-centric and multi-nodular HCC can be attributed to cirrhotic and non-cirrhotic patients, respectively.32 The multi-nodular type has a discernible capsule, is hypovascular, and does not show intrahepatic metastasis and portal vein invasion.42 HCC is a soft tumour except for the fibro-lamellar type. The tumour can be grey-white, tan-brown, yellow due to fatty changes, or green due to bile production.32
Histological Appearance
The International Consensus Group for HCC and the World Health Organization (WHO) proposed the following classifications:
- Early HCC:
a) well-differentiated
b) small size (<2 cm)
c) poorly defined margins, vaguely nodular type - Progressed HCC:
a) >2 cm
b) small size (<2 cm), but moderately differentiated, distinctly nodular type42
Different cytological and architectural patterns can be seen in HCC. In the architectural patterns the most common is the trabecular, which resembles normal liver tissue. The pseudo glandular or acinar type is filled with fibrin or bile and has dilated bile canaliculus-like structures.32,42 Another two types are scirrhous HCC, which is uncommon and shows fibrosis and the compact variant with sinusoid like blood spaces that are inconspicuous and slit-like, giving the tumour a solid appearance.32,43 The cytological patterns include pleomorphic cell, clear cell, sarcomatous change, fatty change, mallory-hyaline bodies, globular hyaline bodies, pale bodies, and ground glass inclusions.43 Pathologists use diagnostic tools, such as immunochemistry, for molecular pathology. This provides more accurate tumour phenotyping and will have a significant role in diagnostic and prognostic purposes.42,44
Precancerous Lesions
Hepatocellular adenoma is a benign tumour composed of cells closely resembling normal hepatocytes and is a rare cause of HCC. It appears more commonly in females using oral contraceptives over 2 years,42,43,45 but also in females with maturity onset diabetes of the young Type 3.45 Other associated diseases with adenoma formation are glycogen storage disease and polycystic kidney.45 The Wnt/β-catenin pathway plays a significant role in the transformation of hepatic adenoma to HCC.38
Dysplastic nodules are hepatocytes <1 mm in diameter with dysplasia but without histological criteria of malignancy.46 The nodules show variable atypia with an increased cell density and are usually <1.5 cm in size with or without fibrous rim and normal nuclear-cytoplasmic ratios.
Large cell dysplasia is characterised by pleomorphic cellular enlargement and multi-nucleation of liver cells that occur in groups or occupy the whole cirrhotic nodule.32,43 Small cell dysplasia is characterised by elevation in the nuclear-cytoplasmic ratio between the normal and diseased liver cell without multinucleation and large nucleoli. It has been assumed that small cell dysplasia has higher tendency of precancerous activity over large cell dysplasia.32,43








