Abstract
Hepatocellular carcinoma (HCC) treatment is variable and depends on the size, location, and presence of extra hepatic metastasis and vascular invasion. HCC treatment options have advanced significantly over the past few decades and include surgical and non-surgical methods. In the past, systemic chemotherapy was the non-surgical treatment and there was no significant increase in overall survival rate. Nowadays sorafenib, a molecular targeted drug, is the treatment of choice and has shown proven benefits in increasing survival time; other systemic therapies did not show longer statistical superiority. However, surgical treatments, such as liver transplantation and surgical resection, are still the only methods offering a curative opportunity; however, these are not free of adverse effects and recurrence of the tumour. Non-surgical techniques including ablative treatment, radiotherapy, transarterial chemoembolisation, and percutaneous ethanol injection also show some benefit in the survival of patients with HCC. Future molecular targeted drugs are currently under investigation in different stages of clinical trials, and there are positive expectations regarding their benefit in treating HCC.
TREATMENT
Surgical Resection
The selection of surgical liver resection takes into consideration the different scoring systems: Barcelona Clinic Liver Cancer (BCLC) scoring system, Milan criteria (MC) (one lesion ≤5 cm in diameter or ≤3 lesions ≤3 cm in diameter), Model of End Stage Liver Disease (MELD), and Child–Pugh. Resection is considered in Child–Pugh Class A and early B and MC and MELD score <9. In Asian countries, indocyanine green (ICG) clearance at 15 minutes with a cut-off >20% is regarded as a contraindication to resection.1 Surgical resection is the treatment of choice for patients with solitary hepatocellular carcinoma (HCC), yet several contraindications are known, such as extra hepatic metastasis, vascular invasion, main bile duct involvement, and bilobar tumour. Patients without chronic liver disease show better long-term results, while patients with Child–Pugh Class A that are selected for surgery will still show postoperative liver decompensation, such as ascites. Continuous ascites for >3 months is a poor prognostic factor. Portal hypertension >10 mmHg is the best indicator of unresolved postoperative hepatic decompensation. Thus, surgical resection should be advised for patients with preserved liver function and without significant portal hypertension.2,3 The extent of the resection should include the removal of all the malignant tissues with maximal preservation of tumour-free liver parenchyma. Limited resection, which tries to preserve liver parenchyma, has a less successful outcome due to tumour recurrence by local spreading; whereas anatomical resection, which is the resection of the vascular territory of the tumour of small solitary HCC lesions, has a higher survival rate than limited resections and is also the treatment of choice.3 The resection goal is to preserve adequate future liver remnant (FLR). Post-hepatectomy liver failure is the most severe complication of hepatectomy and a major cause of death. Several methods have been used in order to assess FLR, and the current method is based on computed tomography (CT) imaging. Hepatobiliary scintigraphy was shown to be superior and have higher prediction in patients with parenchymal disease, such as cirrhosis, cholestasis, steatosis, and chemotherapy injury. The disadvantages of these techniques include the potential discrepancy between the planned and actual transection planes; moreover, it is impossible to predict the functional contribution of liver parenchyma that is poorly perfused or has poor venous drainage after transection. ICG is used to evaluate preoperative liver function reserve and also as an early indicator of outcome following liver resection and orthotopic liver transplant (OLT).4
Small-for-size syndrome can occur in patients following liver resection when the liver regenerative capacity is impaired or as a result of extensive resection. Small-for-size syndrome and post hepatectomy liver failure present similar pathomechanisms, including the reduction of liver mass and portal hyper flow beyond a certain threshold.5 In cirrhotic patients, non-anatomical resections were performed in order to conserve FLR; however, ‘field changes’ in the liver of these patients means they are more prone to tumour recurrence when compared to non-cirrhotic patients.6
According to the Liver Cancer Study Group in Japan, the prognosis of patients after hepatectomy shows 1, 3, 5, and 10-year survival rates of 85%, 64%, 45%, and 21%, respectively, in 6,785 patients followed up between 1988 and 1999.3
Liver Transplantation
OLT in HCC is generally considered to be the best chance of curative treatment for patients with HCC liver dysfunction within the criteria. OLT is also significant in the prevention of postoperative complications associated with liver failure.2,3 Indication in favour of liver transplant (LT) is based on the MC as seen in the Mazzaferro et al.7 study, which showed a 4-year survival of 75% with a recurrence free survival of 83%. This prevents futile transplantation in patients likely to have microscopic extrahepatic metastasis.7 The results of LT in unresectable tumours before the MC were used were poor, with a 3–5-year survival of 15% and a high rate of recurrence in the first months to years after transplantation. The application of the MC has dramatically increased the survival rate and made LT a first-line treatment option for patients with limited tumours.3,8 Recently it has been implied that the MC may be too restrictive. The University of California San Francisco (UCSF) group (San Francisco, California, USA) and the BCLC tumour group, proposed widening the criteria and were supported by the Registry of Tumors in Liver Transplantation (Dallas, Texas, USA). The proposed criteria are one single lesion <6 cm or multiple lesions (no more than 4 lesions), with the largest being ≤5 cm. This expansion shows a 5-year survival of 72%.1,8 Long-term survival after LT for HCC has been shown to decrease due to complications, such as long waiting lists, associated immunosuppressive therapy, graft rejection, and the expansion of the MC. At the time of transplantation, prolonged waiting time for LT has been associated with vascular invasion.3 However, with fewer postoperative surgical complications, the use of marginal grafts, reduced tumour recurrence rates, and improved immunosuppressive regimes, the outcome has improved. Improved postoperative care allows for transplantation in a higher number of patients.3
HCC patients and patients with other underlying liver diseases have been waiting for LT, which makes the LT criteria very strict and competitive; thus, there is an increased interest in using living donors rather than only cadaveric donors.1,3 The living donor liver transplant (LDLT) mortality risk is 0.1% for left hepatectomy and 0.5% for right hepatectomy.1 LDLT increases the number of livers available for transplant, which allows for the extension of transplantations. In patients that do not meet UCSF or MC standards, LDLT should not be performed. Recent studies have shown the survival rate between the cadaveric and LDLT to be similar.1 Comparison between LT and liver resection in HCC is dependent on the patients’ clinical status. In patients with inadequate functional liver parenchyma, LT may be the only curative option. In patients with adequate liver function, liver resection can be the curative option.1,9 The Rahman et al.9 study showed a 5-year survival range of 40–70% in resection and 52–81% in transplantation. Tumour management, while waiting for LT, includes radiofrequency ablation (RFA) and transarterial chemoembolisation (TACE). Liver resection can be used as a bridge to transplantation.3 Downstaging of HCC, using the aforementioned methods, can facilitate LT for patients outside the MC by decreasing the tumour burden. However, HCC recurrence rates after transplantation remain high after downstaging.10
Percutaneous Ethanol Injection
Percutaneous ethanol injection (PEI) therapy has a wide range of anti-tumour effects, is inexpensive, and simple to use. The ethanol is injected by fine needle insertion directly into the mass on consecutive days under ultrasound (US) monitoring. The ethanol will lead to necrosis of the HCC via diffusion, causing thrombosis and ischaemia in the vessels of the tumour. The number of sessions for treatment of HCC depends on the size; for tumours <2 cm the number of sessions is 3–4. For tumour sizes from 2–3.5 cm the number of sessions is 8–12. Up to 4 sessions per week is recommended until arterial devascularisation of the tumour is reached. In non-compliant patients, single sessions are possible, but the injection volume should not exceed 70 mL, thus preventing serious side effects. Confirmation of the treatment efficiency can be detected by CT, angiography, or magnetic resonance imaging (MRI) 24 hours after the procedure.11
The disadvantage of ethanol injection is intense peritoneal pain. The pain can be avoided by slow injection and slow needle removal. Other complications, which occur rarely, are vascular thrombosis caused by ethanol entering the portal vein (PV) and dissemination of the tumour along the needle tract and haemoperitoneum. Small HCC tumours ≤3 cm show 90% necrosis with PEI while larger tumours show lower success rates and high recurrence rates.11
For treatment of larger tumours, it has been suggested to increase the ethanol injected or to use chemoembolisation in combination with PEI. The benefit of these methods is unknown.11 Other methods, for example, the use of acetic acid or hot saline injections and placement of intra-tumoural microwave electrodes, have also been suggested to cause necrosis of the tumour. The advantages of hot saline and acetic acid methods are that they can enter the vascular system without causing damage and they need less volume to embed. Intra-tumoural placement of microwave electrodes shows complete tumour necrosis.2,11 The survival rate with PEI is similar to those of surgical resection in tumours ≤3 cm. In larger tumours, depending on the size, the 1, 3, and 5-year survival rates were 81–97%, 42–82%, and 14–63%, respectively. Recurrence-free survival was not as successful, with rates of 60–83%, 51–82%, and 26–32%, respectively. In microwave ablation (MWA), the survival rate at 1, 2, 3, and 4 years was 45.9%, 26.9, 26.9, and 13.4, respectively. The disease-free survival time was 15.5 months.12 A combination of PEI with TACE has been shown to have a better effect than monotherapy.11
Radiofrequency Ablation
In the RFA method, an electrode is inserted in the tumour under US monitoring, in a percutaneous intercostal or subcostal approach, to start the ablation and cause coagulative necrosis.13,14 RFA is indicated in patients who are not eligible for surgical resection without extra hepatic tumour, Child–Pugh Class A or B, single tumour size ≤5 cm in diameter or three with fewer tumours ≤3 cm in diameter and is considered the preferred method in these patients. RFA is not indicated in patients where the tumour is not visualised by US; the total bilirubin level ≥3 mg/dL; the platelet count is <50×109/L; or prothrombin activity is <50%; there is enterobiliary reflux or adhesion between the tumour and the gastrointestinal tract; there are exophytic or capsular lesions due to complications such as intra-peritoneal bleeding and subcapsular haematomas.15-18
Tumours <3.5 cm, embedded in the hepatic tissue and far from the blood vessels, show the best results. The blood flow interrupts the ablation process by cooling the heating process which makes the tumour adjacent to blood vessels harder to treat.11 Post-ablation US, CT, and MRI can be performed in order to detect residual tumours. The ability of contrast-enhanced US to detect residual tumours 1 day after RFA was 27%. Using positron emission tomography/CT, a residual tumour can be detected 1–2 days after ablation. Non-enhanced T1-weighted imaging (T1W) can show a hyperintense zone 2 days after RFA.13,19,20
Follow-up by US and CT was carried out every 4 months. Levels of serum alpha-fetoprotein (AFP), lectin reactive alpha-fetoprotein, and des-γ-carboxy-prothrombin were measured every month.16 The incidence of complications associated with RFA measured in 2,982 patients was 2.2% per treatment and 1.5% per procedure. A summary of complications displayed in RFA patients is shown in Table 1.16
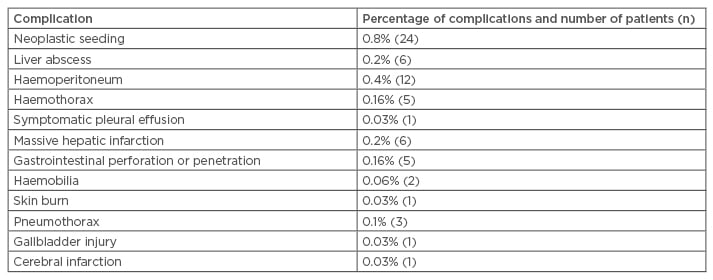
Table 1: Types of complication in 2,982 patients on treatment of radiofrequency ablation for hepatocellular carcinoma.16
In a comprehensive study,12 the survival rates at 1, 3, 5, 7, and 10 years in patients who were i) unsuitable for surgical resection, LT, or refused surgery; ii) free of extrahepatic metastasis or vascular invasion; iii) free of other malignancies that may determine the patient prognosis were 96.6%, 80.5%, 60.2%, 45.1%, and 27.3%, respectively.16 For patients with Child–Pugh Class A or B with MC fulfilled, the 5-year survival rate was 63.8%.16
The distant recurrence rate at 1, 3, 5, 7, and 10 years with no local tumour progression was 25.6%, 63.3%, 74.8%, 78.1%, and 80.8%, respectively. Meta-analyses showed the superiority of RFA over PEI and the correlation to better OS.21 This may be explained by low recurrence and better effectiveness of RFA in necrosis of the HCC.21 When comparing survival between RFA and surgical resection, no significant difference was found.16
Percutaneous Microwave Ablation
MWA is a relatively new method with potentially faster ablation and potentially larger ablation areas. MWA and RFA are considered first-line treatments in HCC. Several advantages over RFA, such as greater penetration of energy into tissues and low sensitivity to physical tissue property variation and impedance, may suggest that MWA could be a better treatment for HCC than RFA.22-26 At 3 months, 6 months, and 12 months post-ablation, patients were followed up by CT scan.27 Successful treatment is the complete absence of contrast enhancement with homogenous hypo-density in the ablation zone.22,27 In patients where MWA did not show favourable results, other techniques can be employed, such as RFA, TACE, PEI, or a combination of ≥2 techniques.22 Complications associated with MWA were reported in Livraghi et al.28 where major complications occurred in 2.9% of patients, including symptomatic pleural effusion, intra-peritoneal bleeding, ileal or colonic perforation, liver decompensation and liver infarction, haemothorax, hepatic abscess, and several other gastrointestinal and cardiac complications. Minor complications were also reported in 7.9% of the patients and included subcutaneous burns, asymptomatic pleural effusion, portal thrombosis, slight thickening of the gallbladder wall, and bradycardia.28 Poggi et al.22 researched patients with small HCC lesions, which showed 100% complete ablation. Intermediate lesions showed 90% complete ablation and large lesions showed 69% complete ablation. Local tumour progression was observed in 5% within a 2-year medium follow-up.
Trans-Arterial Chemoembolisation
TACE is a method in which a chemotherapeutic agent is administered to the hepatic artery with or without lipidol, an iodinated ester that serves as a carrier for chemotherapeutic agents into the tumour, increasing the level of the drug in the tumour, leading to vascular ischaemia and occlusion.14,15
Cisplatin, mitomycin C, and doxorubicin are the most common chemotherapeutic agents used in combination with TACE in HCC, not prioritising one drug over the other.29 TACE is indicated in HCC patients not suitable for resection and with sufficient liver function without extra hepatic metastasis or vascular invasion. Several studies have suggested that TACE may still be used in selected cases with PV thrombosis.30-32 It is also used as a bridge to OLT and prior to, or post, RFA. Contraindications to TACE include: Child–Pugh Class B or higher, renal insufficiency, severely decreased PV flow, and technical difficulties in hepatic intra-arterial treatment.30 Child–Pugh is used as a prognostic factor for survival of patients with unresectable HCC who are treated with TACE.30 Common complications associated with TACE treatment were fever, abdominal pain, vomiting, ascites, and gastrointestinal bleeding.17 Other complications perceived in TACE treatment are liver injury, namely liver abscess, acute hepatic failure, liver infarction; extra hepatic lesions including severe cholecystitis, splenic infarction, pulmonary embolism, gastrointestinal mucosal lesion; iatrogenic injury due to catheter insertion and perforation of the celiac artery and its branches.30 Recently, doxorubicin eluting beads (DEB) were used together with TACE in order to control the drug release duration. DEB can carry several chemotherapeutic agents whilst showing a lower toxicity level to surrounding liver tissue and lower recurrence when compared to TACE alone.29,33
TACE has been shown to increase survival rate in HCC patients.34 The 1, 3, 4, and 5-year survival in a cohort study using TACE-DEB shows 89.9%, 66.3%, 54.2%, and 38.3% survival rates, respectively, with a median of 48.6 months.35 When comparing TACE to surgical resection, no significant difference was shown,36 whereas another study in which sorafenib, a multikinase inhibitor acting on the vascular endothelial growth factor (VEGF) receptor, was combined with TACE showed a superior outcome over TACE alone.37
The Assisted Reproductive Technology (ART) index was developed in order to select the patients that may benefit from retreatment with TACE and those who will not gain any benefit. In this score, patients with a BCLC Stage B and ART score >2.5 points had a poor prognosis, while a score of <2.5 points had a better prognosis of 22.5–28 months OS. Patients with Child–Turcotte–Pugh Class B scoring 7 or 8 points and an ART score between 0–1.5 had a good prognosis as well; 14.5 months OS. This result indicates that patients with an ART index >2.5 may not benefit from TACE retreatment.38 The Hepatoma Arterial-embolisation Prognosis (HAP) score is an index composed of 4 stages developed to select the patients that may benefit from TACE/transarterial embolisation. The majority of the patients in the study had Child–Pugh Class B and MELD >10. Patients with HAP C and D are unlikely to benefit from TACE. The median survival for the groups A, B, C, and D was 27.6, 18.5, 9.0, and 3.6 months, respectively.39
Stereotactic Body Radiation Therapy (SBRT) is a method established >20 years ago. This method can be used in the treatment of patients with a local tumour who are not suitable for RFA, TACE, chemotherapy, or surgery. The appropriate candidates for this therapy are patients with lesions located in a central portal area, regions near to the great vessels, biliary ducts, or metastases located below the diaphragm or at the surface of the liver. The toxicity of Stereotactic Body Radiation Therapy is mainly associated with liver dysfunction, yet other complaints including pain, fatigue, nausea, and vomiting have been reported.40-42
Radioembolisation
Radioembolisation using 90Yttrium (Y90) is a type of brachytherapy which can be used in patients with HCC who fulfil the MC and are in the transplant list. Its effect is mainly to slow down the tumour progression and to decrease the number of patients enlisted in the transplant programme.29,43 It is also useful in patients outside the transplant list and with advanced HCC, by down-staging HCC. In advanced HCC, it is possible to use Y90 due to its low embolic effect. Radioembolisation demonstrates superiority over TACE in advanced stages by downstaging HCC.29,43 Complications are due to irradiation of surrounding tissue and not as a result of the microembolic effect. The most common adverse effect is post-radioembolisation syndrome characterised by nausea and vomiting, fever, fatigue, abdominal discomfort, and anorexia. Other side effects include hepatic dysfunction, biliary damage, gastrointestinal ulceration, lymphopenia, and radiation pneumonitis.43,44 Radioembolisation shows similar survival rates as in the treatment with sorafenib.43 In intermediate stage patients, BCLC-B, the survival rate was 15.4–16.6 months. Finally, in the advanced stage, median OS ranged from 6–10 months.44
Systemic Treatment
Systemic treatment is carried out in two parts. Firstly, by systemic chemotherapy and secondly, by molecular targeted therapy.
Systemic Chemotherapy
Systemic chemotherapy monotherapy with doxorubicin in advanced HCC was the most common therapy before the usage of sorafenib. Other cytotoxic agents, including cisplatin, were given but no favourable outcome was seen and the survival rate was not increased.45,46 Combination therapy of cisplatin with epirubicin, 5-fluorouracil, or doxorubicin, interferon alpha and 5-fluorouracil showed higher responses, but without a higher survival rate.45,46 Hormonal therapies with tamoxifen, octreotide, and interferon were suggested, but a meta-analysis that was conducted did not reveal a significant survival benefit.45 Due to the futility of systemic chemotherapy in improving survival rates, it is not recommended for the treatment of HCC.45,46
Molecular Targeted Therapy
Molecular targeted therapy is the standard treatment in advanced stage HCC.45 The following are some of the treatments being used.
Sorafenib
Sorafenib is a multikinase inhibitor of RAF kinase and VEGF receptors that are involved in the angiogenesis around the tumour. The efficiency of sorafenib was examined in two studies: the SHARP trial47 and Asia-Pacific trial.48 In both studies, survival time in patients with advanced stage HCC was prolonged; the median survival time was <12 months in both studies.45,49
Sorafenib is regarded as safe. Severe adverse effects can be associated with sorafenib, including hand-foot skin reaction, which is the most common side effect, diarrhoea, fatigue, and anorexia.29
Other molecular targeted therapies
Nivolumab, programmed-death-1 (PD-1) inhibitor, gains attention as a future therapy with promising results. Nivolumab demonstrated two complete and seven partial responses with an overall response rate (ORR) of 19%.51 Other molecular therapies with sunitinib, brivanib, linifanib, vandetanib, nintedanib, dovitinib, and a combination therapy of sorafenib with doxorubicin or erlotinib were also studied. The results of the studies showed no benefits in survival rate or time, as compared to sorafenib treatment alone. Sorafenib combination with doxorubicin was the only study to show an increase in median time to progression, OS, and progression-free survival as compared to doxorubicin monotherapy. Synergism between doxorubicin and sorafenib was studied but there was no evidence of synergism.45,52
Other combinations were also studied including: gemcitabin and pegylated doxorubicin, gemcitabin with oxaliplatin and cetuximab, bevacizumab with oxaliplatin and capecitabin, sorafenib and TACE, sorafenib with hepatic resection, and sorafenib with RFA. In the combination of gemcitabine with pegylated liposomal doxorubicin, gemcitabine was administered first because of studies that showed an increase in topoisomerase II expression which leads to an increase in the cytotoxicity.53-56 The treatment was well tolerated by the patients and the outcome showed an ORR of 24%, a disease control rate (DCR) of 58.5%, a progression-free survival of 5.8 months, and a median survival time of 22.5 months.55 Gemcitabine in combination with oxaliplatin, otherwise known as GEMOX, with addition of cetuximab was studied in HCC patients. GEMOX treatment benefits HCC patients because of the lack of liver and kidney side effects. This combination is relatively safe, but some side effects were still documented, such as skin rash, myelosuppression, and neurotoxicity. The outcome of this combination showed an ORR of 20% and DCR of 60%, a median PSF of 4.7 months and an OS of 9.5 months.57,58 Research was conducted to reveal the effect of bevacizumab and capecitabine, in combination with oxaliplatin. The results showed that the combination was well tolerated and the outcome of the results showed a partial response of 20% and DCR of 78%, progression-free survival of 6.8 months and OS of 9.8 months.59
TACE in combination with sorafenib proved to be a useful method of treating HCC. In research undertaken to compare the benefit of sorafenib post-TACE, it was found that for patients administered sorafenib after TACE, the median time to progression did not improve and there was no proven benefit in administering sorafenib after TACE.60 Combination of sorafenib as adjuvant to RFA, or following resection, has not shown any favourable results in terms of recurrence, free survival, time to recurrence, and OS.61
The best supportive treatment for palliative therapy includes pain control, mainly by opiates and with supporting medications against constipation, pruritus, and anti-emetics. Glucocorticoids may be added to alleviate symptoms such as fever, pain, nausea, fatigue, and anorexia. Ascites removal together with albumin supplementation is important in assisting wellbeing. Palliative radiation therapy can be used for distant invasion to lymph node, brain, and especially to the bone. Percutaneous cementoplasty can also be used in case of painful bone metastasis. Nutritional and psychological support together with the methods aforementioned may improve the quality of life in these patients but does not improve life involving a hepatologist, an oncologist, a pain expectancy.62 In order to improve the quality of control specialist, a dietician, a psychologist, and a life a multidisciplinary approach is often needed social worker.








