Abstract
Abnormal liver tests are frequent in patients with inflammatory bowel disease. These may occur at the time of diagnosis or throughout the course of the disease. There are multiple aetiologies, such as concomitant diseases and extraintestinal manifestations of the same disease, primary sclerosing cholangitis being the most characteristic. Other aetiologies include adverse reactions to the drugs used in the treatment of these patients. This review will evaluate the different causes of liver test abnormalities.
INTRODUCTION
Hepatobiliary diseases constitute some of the most common extraintestinal problems of inflammatory bowel diseases (IBD) reported in ulcerative colitis (UC) and Crohn’s disease (CD). Approximately 50% of patients with IBD will present a transient elevation of liver tests during long-term follow up.1 Non-alcoholic fatty liver disease (NAFLD) is the most common cause of impaired liver tests. Primary sclerosing cholangitis (PSC) is a liver disease more specifically associated with IBD, mainly UC.2,3 Other related diseases are autoimmune hepatitis, primary biliary cholangitis (PBC), choledocholithiasis, hepatic amyloidosis, portal vein thrombosis, and drug-induced liver injury (DILI), among others.4 Clinicians must carry out a complete evaluation to determine the aetiology of abnormal liver tests, the possible association with a disease related to IBD, and their clinical relevance. The main objective of this review was to describe the main hepatobiliary manifestations related to IBD.
Liver Function Test Alteration
The transient or persistent elevation of liver tests is frequent in IBD. In a recent study of 306 patients with IBD, 19.6% presented with abnormal liver test results.5 In up to 60.0% of the patients the alterations were mild and spontaneously returned to normal values. The most frequent cause of transient alteration in liver tests is secondary to DILI (34.1%), while fatty liver is the most frequent cause of persistent transaminase alteration and even chronic liver disease (65.4%).1
PRIMARY SCLEROSING CHOLANGITIS
PSC is characterised by chronic inflammation that affects the intra and/or extrahepatic bile ducts. PSC can cause segmental stenosis, saccular dilatations, different degrees of fibrosis, and in advanced stages, even liver cirrhosis.6
Epidemiology
The incidence of PSC is estimated at 0.9 and 0.5 per 100,000 inhabitants per year for North America and Europe, respectively.7 It usually occurs in men, with a median age of diagnosis of 41 years.8 Approximately 50–80% of patients with PSC have concomitant IBD, most often UC. Nevertheless, only 5% of patients with IBD develop PSC. Incidence varies if the diagnosis is radiologic or histopathologic. In a study that included 255 patients with IBD that underwent abdominal surgery and liver biopsy, 12.8% presented findings compatible with PSC. Of these, only 24.1% had alterations of liver function tests.9 In another study of 756 patients with IBD, 24 patients (7.5%) had PSC-compatible lesions in the magnetic resonance cholangiography (MRCP). Only seven (2.2%) of these had a previous PSC diagnosis by clinical findings and/or impaired liver tests. These data suggest that the prevalence may be underestimated, since up to two-thirds of the patients may
be asymptomatic.10
Clinical Findings, Diagnosis, and Complications
Patients can be asymptomatic or present multiple symptoms that can be intermittent, such as fatigue, pruritus, fever, night sweats, and pain in the upper right quadrant. The laboratory finding of a cholestatic pattern (elevation of alkaline phosphatase and γ-glutamyl transferase) is characteristic. The study of choice is MRCP, given it is a non-invasive exam with high sensitivity and specificity.11,12 Typical findings of PSC include multifocal segmental stenosis with saccular dilations, which produce a classic appearance known as ‘string beads’. The European Crohn’s and Colitis Organisation (ECCO) consensus recommends restricting the use of endoscopic retrograde cholangiopancreatography only in cases of need for intervention as an indication for dilatation due to imaging and/or diagnostic cytological findings.13
Liver biopsy is reserved in cases of diagnostic doubt or if small duct PSC is suspected. Characteristic findings are obliterating fibrosis of small bile ducts with concentric periductal fibrosis in an ‘onion skin’ pattern. Causes of secondary sclerosing cholangitis must be excluded, such as infection, immunodeficiency, ischaemia, pancreatic disease, or diseases related to IgG4. The presence of PSC associated with IBD correlates with a specific IBD phenotype.14,15 Loftus et al.16 described a series of 71 patients with IBD. Evidence of backwash ileitis was found in 51% of the PSC-IBD patients compared with 7% in the control group (IBD only). Rectum preservation was found in 52% and 6% of patients, respectively.16 PSC associated with UC mainly presents as extensive colitis beyond the splenic flexion in 90% of cases. There is greater involvement of the right colon, with rectum preservation, frequent reflux ileitis, and increased risk of colorectal carcinoma (CRC), which persists after liver transplantation.5,16 When PSC associates with CD, the course of the IBD tends to be more benign. The predominant phenotype is the inflammatory type of colon, which may or may not have terminal ileum involvement. Stenosing and fistulising phenotypes are less frequent.14,17 The combination of PSC with IBD is associated with complications such as cholangitis, cholecystolithiasis, osteoporosis, fat-soluble vitamin deficiency, and steatorrhoea.18,19 These patients also have an increased risk of developing malignancies, mainly cholangiocarcinoma (CCA) and CRC.10
Primary Sclerosing Cholangitis and Colon Cancer
Patients with IBD have a significantly increased risk of developing CRC, mainly because of the pro-neoplastic effect secondary to chronic intestinal inflammation. The risk is greater if there is an association with PSC.20 In 77 patients with PSC-IBD, CRC was detected in 7.8% versus 2.3% (p=0.016) in the control group (UC without PSC).13 Interestingly, in PSC-UC, all colorectal tumours were located proximal to the splenic flexion. In UC without PSC, tumour location was predominantly in the left colon
(100% versus 40%).
Additional risk factors for the development of CRC include the duration of the disease and the extent of IBD (pancolitis). In a comparative study, 46 of 273 patients with PSC (223 with UC and 50 with CD), it was established that patients with UC had a 56% higher risk of developing CRC compared to CD.21
Primary Sclerosing Cholangitis and Cholangiocarcinoma
The possibility of developing CCA in patients with PSC is 10%. The risk is 400–1,400 times higher than that of the general population,12,22 and is even higher if PSC associates with IBD.23 CCA is mainly associated with intra and extrahepatic PSC, with some cases reported in small duct PSC.24,25 Over half of patients with PSC and CCA are diagnosed at an advanced stage, in part because of the challenges of achieving an early diagnosis. Therefore, the diagnosis of CCA in patients with PSC requires a high index of suspicion and active surveillance.12
Treatment
No therapy has been shown to prevent liver transplantation, CCA, or death.8 The use of ursodeoxycholic acid (15–20 mg/kg/day) is associated with an improvement in the cholestatic pattern. It has not been demonstrated to prevent the progression of the disease: the reason why the American Academy for the Study of Liver Disease (AASLD) does not recommend it.24
Liver transplantation is the only therapy that can cure PSC. Patients with PSC and end-stage liver disease or disabling symptoms (intractable pruritus or repeated cholangitis) should be considered for liver transplantation.13,17
Follow-Up
Due to the higher risk of colorectal cancer, ECCO guidelines12 recommend surveillance colonoscopy in patients with PSC and IBD at diagnosis and every 1 to 2 years after that. Chromoendoscopy with targeted biopsies is the surveillance strategy of choice. In patients with PSC without evidence of IBD, colonoscopy is recommended every 5 years. Screening PSC patients for CCA is a rational approach due to their increased risk of this neoplasia. Ultrasound imaging assessment of the biliary tree (sensitivity: 57%; specificity: 94%) or MRI/MRCP (sensitivity: 89%; specificity: 75%) in combination with CA 19-9 every 6–12 months, seems to be the right approach. Experts recommend MRI for CCA surveillance, as it has higher sensitivity than ultrasound. ERCP should not be considered for surveillance.26,27
OTHER HEPATIC MANIFESTATIONS IN PATIENTS WITH INTESTINAL BOWEL DISEASE
Non-Alcoholic Fatty Liver Disease
NAFLD is a chronic liver disorder, characterised by the presence of steatosis in >5% of hepatocytes. Current data suggest an increase in the prevalence of NAFLD in patients with IBD and is now one of the most frequent hepatic manifestations in IBD. The prevalence of NAFLD is 6.7–35.5% in patients with UC and 7.8–9.5% in CD.28 The range of incidence depends on the diagnostic method used. A controlled study analysed 928 IBD patients, 7.2% had NAFLD diagnosed with abdominal imaging. BMI and prevalence of metabolic syndrome were greater in NAFLD than patients without NAFLD. Risk factors for NAFLD in IBD included small bowel surgery (odds ratio [OR]: 3.7; 95% confidence interval [CI]: 1.5–9.3; p=0.005), hypertension (OR: 3.5; 95% CI: 1.5–8.1; p=0.004), obesity (OR: 2.1; 95% CI: 1.05–4.00; p=0.035), and steroid use (OR:3.7; 95% CI: 1.5–9.3; p=0.005).29
In addition to metabolic syndrome, the pathogenesis of NAFLD in the population with IBD may be more complex and involve specific risk factors for the disease, such as chronic inflammation, drug-induced hepatotoxicity, steroid exposure, malnutrition, and intestinal dysbiosis.30 Nonspecific guidelines for the evaluation of NAFLD in IBD have been established. Ultrasound is commonly used for the screening and evaluation in patients suspected of NAFLD. Non-invasive serum biomarker scores, such as the Fibrosis-4 calculator and NAFLD fibrosis score, have been validated for the assessment of fibrosis. There is also more information about using transient elastography (TE), which may assess the presence of advanced fibrosis.31 A specific treatment for the IBD population has not been evaluated. The current approach to NAFLD therapy is lifestyle and diet modification with the objective of a weight reduction of at least 7%, which has been associated with a biochemical and histological improvement in patients with NAFLD. Pharmacological therapy should be evaluated case-by-case.
Drug-Induced Liver Injury
Drugs used in the treatment of IBD have accounted for some cases of DILI. These may be transient elevations of liver enzymes up to sporadic cases of clinically significant liver injury.32,33 Aminosalicylates report an estimate incidence of 3.2 cases per million prescriptions of DILI, including mild transaminase elevations, cholestatic pattern, and hypersensitivity reactions.34 The hepatoxic effects of thiopurines, azathioprine and 6-mercaptopurine, are primarily mediated by the metabolite 6-methylmercaptopurine.35 In adult patients with IBD starting thiopurines, the American Gastroenterological Association (AGA) have suggested routine thiopurine methyltransferase testing and monitoring of thiopurine metabolite to guide thiopurine dosing if IBD is active.35 Different patterns of hepatocellular, cholestatic, and mixed liver injury have been identified, characteristically being a more acute DILI. Long-term evolution can even lead to liver cirrhosis with portal hypertension secondary to vascular compromise (sinusoidal dilation, sinusoidal obstruction syndrome), and regenerative nodular hyperplasia.36 Methotrexate may cause acute liver test disturbances and prolonged use at cumulative doses greater than 1.5 g may develop macrovesicular steatosis and progressive fibrosis towards cirrhosis. Risk factors for fibrosis with methotrexate use include alcohol consumption, obesity, diabetes, and previous liver disease.37
Anti-TNF have been associated with four types of liver test alterations: 1) infusion hepatitis, which appears after two to five infusions, for which the alteration is usually transient and generally asymptomatic; 2) cholestatic, which can occur later; 3) de novo autoimmune hepatitis, with a hepatocellular pattern and the presence of antinuclear antibodies and other autoantibodies; and 4) reactivation of chronic hepatitis B, being necessary to test it before starting biological therapy.5,38 Vedolizumab is a gut-specific anti-integrin that binds α4-β7 to MAdCAM1. In a systematic review it was shown that liver test alterations were not significant compared to placebo.39 Small molecules are an effective treatment in moderate-to-severe, immune-refractory or anti-TNF-failing UC. No significant alterations in liver tests have been reported regarding these.39 Table 1 shows the different hepatic damage patterns associated with drugs used in the treatment of IBD patients.
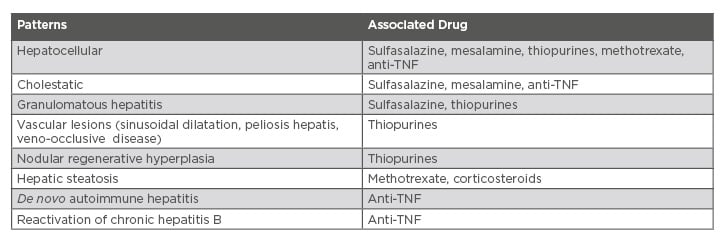
Table 1: Hepatic damage patterns associated with drugs for intestinal bowel disease.
Primary Biliary Cholangitis
PBC is a chronic cholestatic nonsuppurative destructive cholangitis. Most patients are asymptomatic or have nonspecific symptoms such as fatigue or pruritus and it is not usually associated with IBD. Liberal et al.40 described a series of six patients with PBC-IBD where the majority were women without differences between UC or CD.40
Autoimmune Hepatitis
Autoimmune hepatitis (AIH) is a chronic liver disease characterised by alteration of transaminases, hypergammaglobulinaemia, and periportal hepatitis in liver biopsy. The prevalence in patients with IBD is low. It has been reported mainly in children with UC reaching up to 0.77%. In patients with PSC, the prevalence is higher, where the overlap of AIH was observed in 10% of cases.41,42 The response to the treatment of AIH is not affected by the presence of IBD.
Pyogenic Liver Abscess
Patients with IBD have a higher risk of pyogenic liver abscess than the general population. In a cohort study, the incidence was higher in IBD patients (6.72 IBD versus 4.06 per 10,000 person-year in non-IBD).43 The abscesses are often multiple in number and are located more frequently in the right hepatic lobe. Clinically they present with abdominal pain, jaundice, fever, diarrhoea, and in some cases, hepatomegaly. They are mainly associated with CD due to transmural inflammation and may be secondary to direct extension of intra-abdominal abscess, pylephlebitis, or secondary to fistulising disease. Additional risk factors are diabetes and bile duct manipulation.44 Treatment does not differ from management in other clinical contexts. A guided antibiotic therapy should be administered according to cultures or drainage results, size, and evolution.30
Hepatic Amyloidosis
Secondary hepatic amyloidosis is a rare complication that has been reported in 0.90% of patients with CD. It is mainly described in cases of severe CD with infectious complications and intestinal resection, and in 0.07% of patients with UC.45 Chronic inflammatory activity in the intestine contributes to the deposition of amyloid in the vessels and sinusoids of almost any organ, including the liver, which leads to asymptomatic hepatomegaly. The treatment is to decrease IBD activity.
Granulomatous Hepatitis
Granulomatous hepatitis is a rare complication of IBD, with a prevalence of less than 1%, which is more frequent if associated with CD. It can be induced by drugs used in IBD treatment, such as mesalamine and sulfasalazine, and also with other concomitant autoimmune pathologies such as PBC and AIH. The presence of non-calcified granulomas characterises it, occasionally with multinucleated cells, which are located both in the portal space and the lobules. Patients are usually asymptomatic, so suspicion should arise in the presence of a cholestatic pattern.46
Portal Vein Thrombosis
Patients with IBD have a known risk of increased thromboembolic disease, and the portal vein is a common place of thrombosis. Major risks have been described in the postoperative period of IBD and during exacerbations, although it may occur in patients in remission. In a Spanish retrospective study, 40% of patients who presented thrombotic episodes also had a proven prothrombotic genetic factor, the most frequent being hyperhomocysteinaemia.47 Therefore, the ECCO guidelines recommend an appropriate evaluation for both the underlying acquired prothrombotic conditions (related to IBD) and for hereditary thrombophilia.12 Treatment with anticoagulants is recommended according to general guidelines.
CONCLUSION
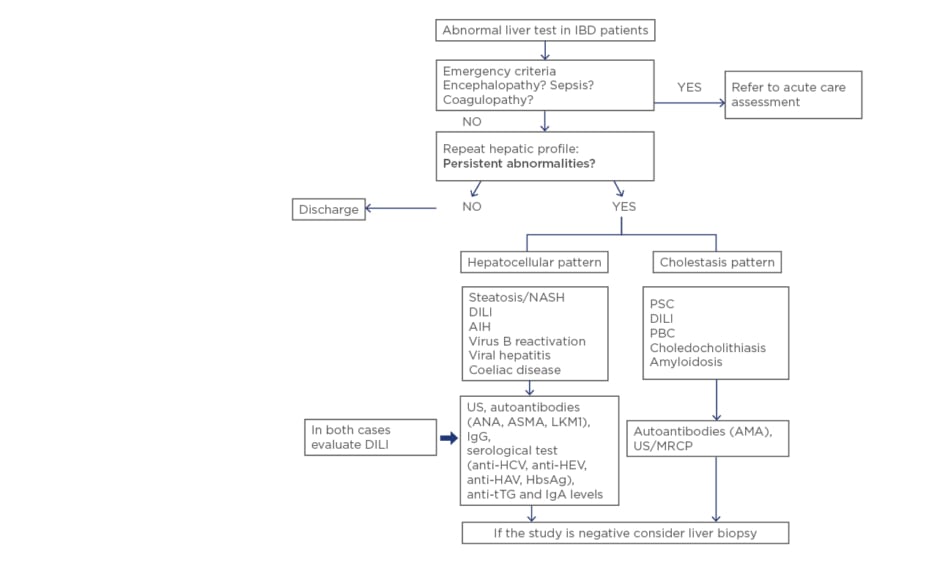
Elevation of liver enzymes is frequent in patients with IBD. Causes are varied and alterations range from slight increases to progressive severe diseases with poor prognosis. Therefore, in patients with IBD, liver tests should be routinely monitored, and a full diagnostic workup performed if they are altered (Figure 1). Differential diagnosis should always include DILI.
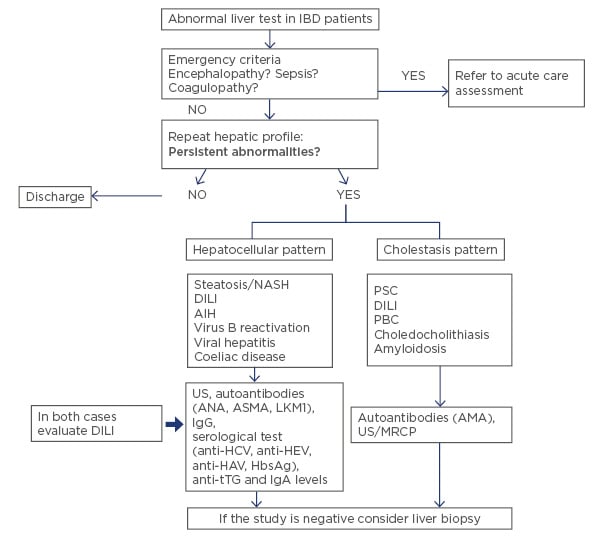
Figure 1: What should we do with abnormal liver test in inflammatory bowel disease? A stepwise approach for patients with abnormal liver test in IBD.
AIH: autoimmune hepatitis; ANA: antinuclear antibody; ASMA: anti-smooth muscle antibody; LKM1: liver kidney microsome antibody; AMA: antimitochondrial antibody; PBC: primary biliary cholangitis; DILI: drug-induced liver injury; HAV: hepatitis A virus; HCV: hepatitis C virus; HEV: hepatitis E virus; IBD: inflammatory bowel disease; MRCP: magnetic resonance cholangiography; NASH: non-alcoholic steatohepatitis; PSC: primary sclerosing cholangitis; tTG: tissue transglutaminase; US: ultrasound.