Abstract
Portal vein thrombosis (PVT) is considered a common complication of liver cirrhosis. Its prevalence increases with liver disease severity, reaching 25% in patients awaiting liver transplantation (LT). The majority of patients with cirrhosis are diagnosed incidentally with PVT during routine ultrasound in their cirrhosis follow-up. Doppler ultrasound is the recommended first-line investigation. Computed tomography or magnetic resonance angiography are the best methods to assess the extent of the PVT. The natural history of PVT in liver cirrhosis is not very well defined, but in the context of LT the deleterious effects of PVT are better known. There are no consensus guidelines about the treatment of PVT in cirrhotic patients and although anticoagulation is considered as the first-line therapy, the evidence regarding this treatment is based on a small series of patients. Nonetheless, it seems that anticoagulation therapy is useful in cirrhotic patients with PVT, particularly in patients who are candidates for a LT, in order to maximise the recanalisation rate and prevent thrombus progression. This treatment must be administered as soon as possible following a prophylactic treatment to avoid variceal bleeding, otherwise it seems to have a broad safety profile. A transjugular intrahepatic portosystemic shunt would be the alternative procedure for patients with no response to anticoagulation therapy or where portal hypertension complications occur.
INTRODUCTION
The portal vein is an 8 cm conduit that originates from the confluence of the superior mesenteric and splenic veins posterior to the neck of the pancreas. It accounts for 75% of the blood supply to the liver.
Portal vein thrombosis (PVT) is an obstruction of the portal vein trunk and/or its branches by a blood clot, which includes the splenic, superior mesenteric, and inferior mesenteric veins. It can present in a variety of conditions, including cancer, infections, myeloproliferative diseases, inflammatory conditions, following ablative therapy for hepatocellular carcinoma (HCC), and cirrhosis.1 From a clinical point of view, there are two types of PVT:
- Acute: sudden formation of a thrombus within the portal vein, which was not detected during the previous biannual ultrasound. Occlusion may be complete or partial
- Chronic (portal cavernoma): replacement of the normal portal vein by a network of hepatopetal collateral veins. It functions as a portoportal shunt2
Portal Vein Thrombosis and Cirrhosis
To date, there has been no published consensus on management of non-malignant PVT in liver cirrhosis. Moreover, there have only been a few studies about the incidence and prevalence of PVT in cirrhosis. In any case, PVT is the most common thrombotic event in cirrhotic patients. The prevalence is between 0.6% and 26%, depending on the method used for its evaluation.3-5 A large, observational, prospective study in Italy, estimating the prevalence of PVT evaluated by ultrasound with power-Doppler in a cohort of patients with liver cirrhosis of any aetiology and severity, is ongoing and will provide useful clinical data.6 A recent prospective study found a cumulative incidence of PVT of 4.6%, 8.2%, and 10.7% at 1, 3, and 5 years, respectively.7 Other studies that included patients with more severe cirrhosis at baseline found a higher incidence (7.4%, 12.8%, and 16.4% per year).8-10 In spite of previous data, there are no specific recommendations regarding early detection of PVT in cirrhosis aside from the screening of HCC.
In cirrhotic patients the pathogenesis of PVT appears multifactorial. Slow blood flow in the portal venous system increases the probability of developing thrombi,9 but the reproducibility of portal vein flow velocity measurements between different equipment and operators make it difficult to find absolute values.
It is now recognised that cirrhosis is associated with the hypercoagulability of plasma. In these patients, plasma is partially resistant to anticoagulation mediated by thrombomodulin. This situation is probably the result of two common alterations in cirrhotic patients: elevated levels of factor VIII (a procoagulant driver) in combination with decreased levels of protein C (an anticoagulant driver).11
Systemic thrombotic risk factors such as factor V Leiden mutation and the G20210A prothrombin mutation may play a role in the formation in PVT, however there are some contradictory results in the area.7,12 Other risk factors for PVT include endoscopic sclerotherapy of oesophageal varices,13 gastrointestinal infections,14 and bacterial translocation/endotoxinaemia, which might be mitigated by with enoxaparin.15
DIAGNOSIS
Most patients with cirrhosis are diagnosed with asymptomatic PVT during routine ultrasound. The sensitivity and specificity of Doppler ultrasound are 89% and 92%, respectively,16 so it is the primary method of choice in this context. If Doppler ultrasound shows portal vein patency, no further studies are indicated. However, ultrasound limitations can cause a false positive result, for example due to their accuracy being clearly influenced by the operator skill,17 or very low flows.18 The main ultrasound findings of PVT include hyperechoic material within the vessel lumen, absence of flow, and an inability to identify or dilate the portal vein. When portal cavernoma has developed, tortuous vessels in the porta hepatis are present.19
It is important (from a prognosis and treatment point of view) to distinguish between partial and complete PVT. In occlusive PVT, a thrombus leaves no channel for blood flow. Contrast enhanced ultrasound is more sensitive than Doppler ultrasound, since it can visualise hypoechoic or small thrombi (≤3 mm).20 Furthermore, during the arterial phase, contrast enhanced ultrasound allows identification of the malignant thrombus origin when there is vascularisation (the thrombus appears hyperechoic).21,22
Enhanced computed tomography and magnetic resonance imaging are the best methods to assess the extent of the PVT. In addition, they provide information about the development of collateral circulation, the status of adjacent organs, and are indicated if intestinal ischaemia or HCC are suspected.2
CLINICAL PRESENTATION AND NATURAL HISTORY OF PORTAL VEIN THROMBOSIS
In patients with cirrhosis, diagnosis of PVT is growing in relation to the increased frequency of liver imaging. The most common scenario is therefore the detection of asymptomatic PVT during routine ultrasound examination. Complications of PVT include variceal bleeding, failure of endoscopic control of bleeding, intestinal ischaemia (in patients with extension of the thrombus into the superior mesenteric vein), and portal biliopathy (causing partial or complete bile duct obstruction).5,14,23
The risk of developing PVT increases with the severity of the liver disease, although the development of PVT is a marker rather than a direct cause of cirrhosis progression.7 On the other hand, some studies show that PVT has little influence on prognosis and is not associated with an increased risk of death or reduced chance of undergoing transplantation.23,24 A recent meta-analysis concluded that PVT appears to increase mortality and liver decompensation from ascites, but the small number of included studies limited widespread conclusions.25 It is likely that the prognosis of cirrhosis with PVT will be influenced by the degree of occlusion of the venous lumen and the clinical situation of the patient at the time of diagnosis.
Natural history studies have identified relatively high rates of recanalisation in cirrhotic patients with non-malignant PVT. In two different reports, PVT improved in 47.6% and 45% of patients, respectively.23,26 In another recent study, the spontaneous repermeation rate was even higher (70%).7 In all three studies most patients only had a partial thrombosis, which could have influenced these results, as stated before. Nonetheless, these data show that in a percentage of patients thromboses will progress. If a simultaneous worsening in the liver function exists, the patient could be a candidate for liver transplantation (LT); in this context, the deleterious effects of PVT are better understood.
Portal Vein Thrombosis and Liver Transplantation
PVT may adversely affect the outcome of LT but currently it is considered only a relative contraindication for LT.27,28 The prevalence of PVT in patients awaiting LT has a very broad range (between 2.1% and 23.3%). PVT presents important challenges in patients undergoing LT due to the technical requirements of clot removal or alternate vascular reconstructions.29 The Yerdel classification29 is the most widely accepted in defining not only the morphology of PVT, but also the presence of suitable collateral vessels that could be useful for an extra-anatomical reconstruction of portal flow; allowing appropriate graft selection and planning of the transplant surgical procedure.
The impact of PVT on LT has not been clearly defined, probably because most studies are retrospective and only some of them evaluate patients with occlusive and non-occlusive PVT separately. Again, the separation between occlusive and non-occlusive thrombosis is very important; in patients with partial PVT, post-transplant mortality outcomes are no different from non-PVT patients but it is significantly increased in patients with complete PVT.13,30 Data from a high volume centre suggests that PVT is an independent predictor of mortality post LT, with occlusive PVT conferring an additional increase in mortality at 30 days.31
PVT following LT carries a poor prognosis. The rate of de novo PVT occurrence post-LT is 0–2%.29 On the other hand, patients with PVT at the time of LT also have a higher risk of recurrent PVT after transplantation (rates of 2–3%).32 In the case of transjugular intrahepatic portosystemic shunt (TIPS) insertion for the treatment of PVT, the incorrect positioning of its distal tip must be avoided due to its possible interference with LT.
For all the reasons noted above, the treatment of cirrhotic patients with PVT seems especially appropriate in candidates for LT, so they can achieve a complete/partial recanalisation or at least prevent extension of thrombus, particularly to the superior mesenteric vein.
TREATMENT
There is no consensus about the standardised treatment of PVT in cirrhotic patients. How to choose the best candidate, the best moment, and the best type of treatment are questions without a clear answer, because the natural history of PVT in cirrhosis is still a matter of debate.33 A reasonable decision in this context could be made on a case-by-case basis,34 but it would be desirable to establish a model for the general management of these patients. There are several therapeutic strategies in patients with cirrhosis and PVT: anticoagulation, TIPS, and thrombolytic therapy.
ANTICOAGULATION
In spite of the reservations that will be noted below, anticoagulation is the first-line therapy in cirrhotic patients with PVT. There are seven studies published to date that have evaluated this treatment in these patients.
Primary Prevention
Villa et al.15 performed a randomised controlled trial to evaluate the results of enoxaparin in preventing PVT in patients with advanced cirrhosis. There was no thrombosis in the active arm (n=34 patients) compared with 27.7% PVT in the control arm (n=36 patients), with a follow-up of 2 years. No relevant side effects or haemorrhagic events were observed. Though several limitations of this trial have been identified,35 a recent study with cirrhotic rats showed that prolonged administration of enoxaparin improves portal hypertension and liver cirrhosis, probably by potentiating fibrosis regression. These results could help to explain the results of the study of Villa et al.15 In any case, before the prophylactic use of anticoagulant therapy is recommended in cirrhotic patients, further confirmative studies are required.
Secondary Prevention
It is likely that anticoagulation treatment is frequently used in clinical practice, but there are only scarce data about its results. In Table 1 the six studies published are shown (including a total of 193 patients). Only a few patients had a portal cavernoma. Treatment (warfarin and/or low-molecular-weight heparin [LMWH]) was associated with complete recanalisation rates between 36% and 75%. Thrombus progression was reported between 0% and 15%.
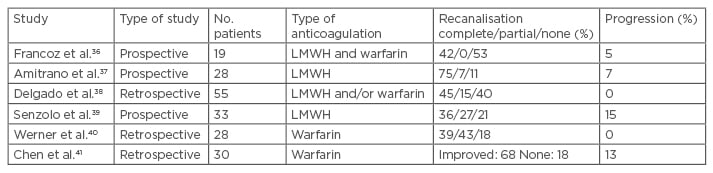
Table 1: Summary of studies about the use of anticoagulation therapy with secondary prevention in patients with portal vein thrombosis and liver cirrhosis.
LMWH: low molecular weight heparin.
In two of these studies, the early administration of anticoagulation was associated with a greater probability of recanalisation.38,39 In the three studies compared treatment with control subjects, the recanalisation and thrombus progression rates were favourable to the treated group.36,39,41 Amitrano et al.37 and Delgado et al.38 reported re-thrombosis rates after stopping anticoagulation between 27.2% and 38%. These results outline the possibility of maintaining anticoagulation therapy in the long-term at least for those patients awaiting LT.
Only two patients developed severe bleeding complications: one cerebral haemorrhage and one significant vaginal bleeding.39,40 Delgado et al.38 reported five bleeding complications, probably in relation to therapy, and identified a platelet count <50×109/L as the only parameter significantly related to a higher risk of bleeding. Chen et al.41 reported four other severe bleeding events. Of these 11 patients, 10 were treated with vitamin K antagonists (VKA) alone. As previously suggested, this fact could support a greater safety profile of LMWH.42 On the other hand, a recent study in a group of cirrhotic patients with upper-gastrointestinal bleeding reported that anticoagulation therapy is not necessarily associated with an increased morbidity/mortality.43 No other significant side effects were observed during the treatment. No deaths were found to be associated with this therapy.
All the patients must be screened to grade varices prior to receiving anticoagulation therapy. There is also no consensus on the prophylactic treatment of variceal bleeding in these patients, but it seems logical that it must follow recognised international guidelines.44
Our group recently published results related to 27 cirrhotic patients with non-malignant PVT. Twenty-six patients received anticoagulation: 23 LMWH and 3 VKA. The median time from diagnosis to the initiation of treatment was 2 weeks. The complete recanalisation rate was 57.6%. The median time to achieving this complete response was 10 months (95% confidence interval: 3–17). Re-thrombosis occurred in 35.7% of patients. Patients with no response to treatment did not show progression of thrombosis. Only two patients, one of them with 30,000 platelets, presented a bleeding complication (mild in both cases). No significant differences regarding the appearance of portal hypertension related complications were observed. Patients with a MELD score <8 achieved recanalisation within a significantly shorter timeframe compared with the other patients (p=0.04).45
The VKA should be used with regular laboratory monitoring. Though cirrhotic patients are usually treated with doses aimed at 2.0–3.0 international normalised ratio, this value might not be representative of the real anticoagulation.46 LMWH does not seem to require laboratory monitoring to adjust dosage. In any case, the anti-Xa assay in cirrhosis is not representative of the real anticoagulation.46 A thrombin generation test might be considered for monitoring the anticoagulation effect in this group of patients, but this needs to be evaluated.39
Newer oral anticoagulants represent an attractive option for cirrhosis patients due to ease of administration, and although hardly studied in this population, a recent publication comparing their safety to traditional anticoagulation displayed similar rates of bleeding in a cohort of selected cirrhosis patients.47 However, due to the predominant hepatic metabolism of these agents, their use is not advisable in decompensated cirrhosis.
Taking into account all of the above, although further controlled studies with more patients are clearly needed, it seems that anticoagulation therapy is useful in cirrhotic patients with PVT, particularly in patients who are candidates for a LT, in order to maximise the recanalisation rate and prevent thrombus progression. This treatment is most efficacious when administered as early as possible and it seems to have a broad safety profile. In this sense, LMWH appears to be safer, but at present it is not possible to recommend a specific type of LMWH or its optimal dosage in order to set up liver-specific guidelines. There is not a defined regularity of ultrasound surveillance to monitor PVT across the duration of therapy, but a reasonable interval would be every 3 months.
TRANSJUGULAR INTRAHEPATIC PORTOSYSTEMIC SHUNT
The reported technical success rate for TIPS in cirrhotic patients with PVT is 75–100%.48-52 However, many of these studies are retrospective and the indication for TIPS was the treatment of portal hypertension complications but not PVT itself. Treatment with TIPS may be feasible if portal cavernoma is present but is not an option if a patent intra hepatic portal vein branch is lacking.48
The role of anticoagulation therapy in post-TIPs patients remains undefined. A recent trial shows that anticoagulation may not be necessary in certain patients with PVT and the presence of a superior mesenteric vein thrombus may be used to predict recanalisation failure.53
The procedure-related complication rate is 0–17%35 and the two main postoperative complications are the risk of developing hepatic encephalopathy and shunt dysfunction, with incidences of 7–32% and 0–50%, respectively.54 These incidences have, however, been reduced with the use of covered stents, and the long-term outcome of TIPS in cirrhotic patients with PVT is excellent.49
THROMBOLYSIS AND PERCUTANEOUS PORTAL VEIN RECANALISATION
Thrombolytics (tissue plasminogen activators) can be infused into the portal vein indirectly (injection into the superior mesenteric artery through the femoral or radical artery) or directly (via a percutaneous transhepatic or transjugular intrahepatic approach).14,55 PVT can be recanalised percutaneously with balloon angioplasty or by the placement of stents.56 Even though these procedures may be effective in patients with cirrhosis and PVT, experience is scarce and complications may be serious.
CONCLUSION
Diagnosis of PVT is occurring more frequently in patients with cirrhosis (above all, in those with more severe liver disease) because of the increasing use of ultrasound in their follow-up. Doppler ultrasound is the recommended first-line investigation in this context. When PVT is identified the use of computed tomography and magnetic resonance imaging should be considered to rule out associated HCC.
There are no clinical guidelines regarding treatment of PVT in cirrhotic patients. However, patients with cirrhosis and occlusive PVT, LT candidates, or those with an evident thrombus progression, should receive anticoagulation therapy, either long-term or until LT. This therapy should be administered as soon as possible but only following prophylactic treatment of oesophageal varices. TIPS would be the best alternative procedure if anticoagulation therapy is ineffective or if hypertension portal complications occur.
If PVT is diagnosed when cavernoma is already present, anticoagulation therapy would only be indicated for patients with a thrombophilic disorder or in cases of thrombus progression, mostly in candidates for LT. Though PVT is a risk factor for LT, it is not considered an absolute contraindication even when PVT is complete. At this point, on the basis of all considerations and taking into account the published studies, we propose an algorithm for management of PVT (Figure 1).
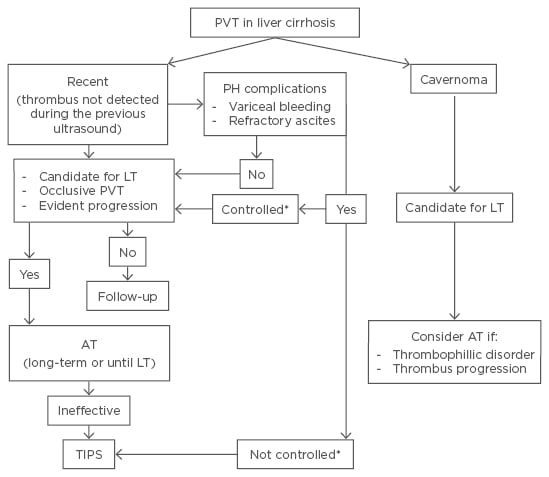
Figure 1: Algorithm for treatment of non-malignant portal vein thrombosis in liver cirrhosis.
PVT: portal vein thrombosis; LT: liver transplantation; AT: anticoagulation therapy; TIPS: transjugular intrahepatic portosystemic shunt; PH: portal hypertension.
*Controlled or not by endoscopic therapy, paracentesis, drugs.
In our opinion, specific guidelines about management of PVT in cirrhotic patients should be established in the near future. It seems clear that there is a lack of randomised control trials regarding management of PVT, mainly pertaining to the role of anticoagulation in these patients, not only about its efficacy, but also its safety and laboratory monitoring. With respect to the possible benefits of primary prophylaxis, it will be necessary to confirm initial findings and perhaps define the target population (all the cirrhotic patients, independent of liver function, prioritise those patients with slow portal flow, etc.).
Moreover, recent studies have shown that the activation of the coagulation factors may stimulate fibrogenesis.57 This same mechanism could, simultaneously, lead to the occurrence of PVT, as mentioned earlier. In the area of research done by Villa et al.15 it would be desirable to confirm the role of anticoagulation therapy in the prevention not only of the development of PVT but also its effect on worsening of liver disease.








