Abstract
Acute-on-chronic liver failure (ACLF) is a syndrome that describes acute decompensation of chronic liver disease with differing definitions worldwide, but is universally associated with high short-term mortality. This is becoming increasingly recognised as a unique entity that affects both adults and children. This narrative review summarises the current available evidence from paediatric studies on definition, incidence, pathophysiology, and outcome, with reference to data on ACLF from adult literature. Paediatric data remain scarce, and study groups have used differing inclusion criteria that have limited generalisability of data. There is a crucial need for a consensus definition for paediatric ACLF so that future collaborative research may provide better understanding on the epidemiology, pathophysiology, risk factors, and outcome of this clinical entity.
Key Points
2. While the only possible treatment for paediatric ACLF is transplantation, the condition can be supported through therapies to manage hepatic and extrahepatic complications.
3. Studies have shown the importance of monitoring ACLF in order to prevent short-term mortality through emergency transplantation in a ‘golden window’ of time.
INTRODUCTION
There is growing recognition of acute-on-chronic liver failure (ACLF) as a distinct clinical syndrome that portends excessively high short-to-medium term mortality rates in patients with chronic liver disease (CLD).1 ACLF is characterised by severe, acute hepatic decompensation in patients with underlying CLD, with or without cirrhosis, resulting in liver failure and failure in one or more extrahepatic organs, and associated with increased risk of death within 28 days–3 months from onset. The definitions proposed by the Asian Pacific Association for the Study of the Liver (APASL)2 and the European Association for the Study of the Liver–Chronic Liver Failure (EASL-CLIF) Consortium3 are most frequently used in studies on adult patients. However, it remains that there is no universally accepted consensus on the definition or diagnostic criteria for ACLF. Moreover, paediatric data is significantly lacking, and ACLF in children remains poorly defined. In recent years, there has been greater awareness and interest in this topic, and new studies have emerged that provide better understanding of ACLF in paediatric patients with CLD.
In this review, the authors aim to highlight and summarise current and latest evidence on definition, epidemiology, pathophysiology, management, and outcome of ACLF in children.
DEFINITION AND DIAGNOSTIC CRITERIA
There are different definitions proposed by various international hepatology societies, with the main distinction being the inclusion of extra-hepatic organ failure as a major criterion (Table 1). The definition provided by the APASL in 2009,2 and subsequently updated in 20144 and 2019,5 characterises ACLF as an acute hepatic insult manifesting as jaundice and coagulopathy complicated within 4 weeks by ascites and/or hepatic encephalopathy (HE) in a patient with previously diagnosed or undiagnosed chronic liver disease (CLD) or cirrhosis.Notably, the APASL definition excludes patients with prior decompensation, focuses primarily on liver dysfunction, and considers only intrahepatic insults as precipitating factors, and extrahepatic factors such as bacterial infections as a consequential complication.
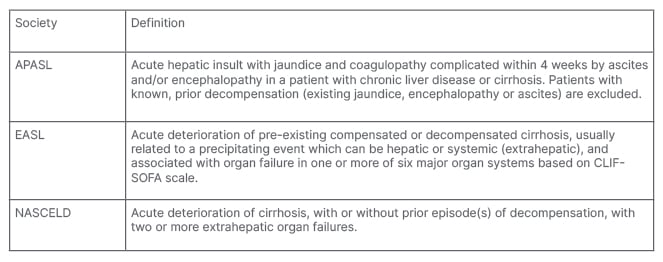
Table 1: Comparison of current definitions for acute on chronic liver failure.
APASL: Asian Pacific Association for the Study of the Liver; EASL: European Association for the Study of the Liver; NASCELD: North American Consortium for the Study of End Stage Liver Disease.
On the other hand, the European Association for the Study of Liver–Chronic Liver Failure (EASL–CLIF) consortium defined ACLF in the CANONIC study in patients with compensated cirrhosis and/or prior episode(s) of decompensation based on the failure of one or more organs including the liver, and included patients with hepatic and/or non-hepatic insults.3 The CLIF-SOFA (sequential organ failure assessment) and its simplified version, CLIF Consortium Organ Failure (CLIF-C OF) scores are calculated based on severity of organ failure in hepatic, renal, neurologic, haematology, circulatory, and respiratory systems, and mortality in ACLF correlates with the ACLF grade that is based on number of organ failures.6 In the EASL-CLIF definition, precipitating disorders may include intrahepatic, extrahepatic (including infection, gastrointestinal haemorrhage), or both.
Similarly, the North American Consortium for the Study of End-Stage Liver Disease’s definition of acute-on-chronic liver failure (NACSELD-ACLF)7-9 involves acute deterioration of cirrhosis with failure in two or more extrahepatic organs.
Although these definitions have been derived from adult populations, they have also been adapted for use in children by various paediatric groups. The APASL definition was used as a basis for several paediatric studies,10,11 whereas Godfrey et al.12 and Bolia et al.13 adapted the CLIF-C OF and CLIF-SOFA scores by modifying creatinine derangements and classifying cardiorespiratory failure according to age-appropriate cut-offs. One recent North American single-centre study by Banc-Husu et al.14 identified paediatric ACLF cases using the NACSELD-ACLF criteria.
With such heterogeneity in definitions and inclusion criteria, it is virtually impossible to generalise the findings and draw meaningful conclusions from current paediatric studies. In the updated 2019 APASL consensus,5 major limitations were acknowledged in existing definitions derived from adult populations, among which clinical identification of HE and ascites may often be difficult in young children, and some paediatric liver diseases can present with hepatic failure without significant hyperbilirubinaemia. There is a pressing need to develop and validate the definition of paediatric ACLF to allow early identification and treatment, accurate prognostication, and facilitate global collaborative research efforts.15,16
PATHOPHYSIOLOGY
The concept of ‘predisposition, injury, response, organ failure’ (PIRO) in explaining the pathophysiological basis of ACLF has been very elegantly described by Jalan et al.17 in their 2012 review paper. To briefly summarise this concept, predisposition refers to the underlying chronic liver disease or cirrhosis and severity of hepatic dysfunction and extra-hepatic organ involvement. A precipitating injurious event such as drug-induced liver injury, superimposed viral hepatitis, variceal bleeding, or sepsis may then exacerbate liver injury, leading to acute decompensation in hepatic function. An abnormal systemic inflammatory response syndrome ensues which may then lead to an over-compensatory anti-inflammatory response and ‘immune paralysis’ in both the innate and adaptive immune systems. Although the exact mechanism of this immune dysregulation is unclear, infections are recognised as common triggers and complications of ACLF, which further heighten the pro-inflammatory response in a vicious cycle and are associated with complications such as HE, renal dysfunction, rebleeding and increased mortality. Ongoing infection, endotoxaemia, pro-inflammatory state and systemic inflammatory response syndrome result in dysregulation of systemic and hepatic haemodynamics, each factor variably contributing to end-organ dysfunction including decreased hepatocyte function with cholestasis and coagulopathy, HE and cerebral oedema, acute kidney injury, subclinical myocardial injury and circulatory failure, and acute respiratory distress syndrome.
EPIDEMIOLOGY
Epidemiological data on ACLF in children are limited to single-centre studies using different inclusion criteria, and comprising very heterogeneous patient groups. The incidence of ACLF, as defined by APASL criteria, was reported by Lal et al.11 and Alam et al.18 to be 11–14% of paediatric patients with chronic liver disease from two single-centre studies in India. A single-centre study from the USA using an adapted NACSELD reported an incidence of 14% among 144 children with chronic liver disease listed for transplant, and found that ACLF accounted for 12% of hospitalisations for decompensated cirrhosis.14 By contrast, using a modified paediatric-CLIF definition, ACLF made up 2.3% of all children listed for liver transplantation on the Organ Procurement and Transplantation Network.12 There has also been published data on incidence of ACLF among specific patient sub-populations. For example, ACLF, diagnosed based on EASL-CLIF criteria, was reported to occur in 20% of patients with biliary atresia, which is the most common cause of chronic liver disease in children.19 Other studies have also found that ACLF accounted for nearly 18% of patients with liver cirrhosis admitted to the paediatric intensive care unit.20
In the adult population, the incidence of ACLF similarly differs based on the definition used. A study of 72,316 patients with decompensated cirrhosis has shown that the prevalence of ACLF was 26.4% and 9.8% based on the European and North American definitions, respectively.21,22 In another study of 80,383 adult patients with cirrhosis, 783 patients developed ACLF that fulfilled both EASL-CLIF and APASL criteria; 4296 developed EASL-CLIF ACLF alone; and 574 developed APASL ACLF alone. The overall incidence rate of ACLF in adult patients with liver cirrhosis using APASL criteria was 5.7 per 1,000 person-years, whereas the incidence rate of ACLF using EASL-CLIF criteria was 20.1 per 1,000 person-years.23
UNDERLYING AETIOLOGY OF CHRONIC LIVER DISEASE
While alcoholic cirrhosis, non-alcoholic steatohepatitis, and chronic hepatitis B have been reported consistently as the predominant aetiologies of chronic liver disease in adults,3,7-9 the aetiology of primary liver disease in children with ACLF is more varied among studies. Paediatric studies from Asia using the APASL criteria have found Wilson’s disease (41–52%) and autoimmune liver disease (13–42%) to be the most common underlying liver disorders, followed by viral hepatitis B accounting for around 5.6–6.5%.10,11,18,24 These were in contrast to findings from a limited number of North American studies that have used the adapted EASL-CLIF and NACSELD criteria for ACLF, which found biliary atresia to be the predominant aetiology of chronic liver disease, accounting for approximately half of cases that presented with ACLF.12,14 This disparity may be explained with the following reasons. The APASL definition includes patients with only intrahepatic insults, such as acute flare of underlying liver disease and superimposed or reactivated viral hepatitis; hence, this may explain the higher representation of autoimmune hepatitis and Wilson’s disease, as well as viral hepatitis triggers in the Asian cohorts. To support this point, Jagadisan et al.25 explained in their study that the small number of biliary atresia cases in their series was due to the the premature follow-up of young patients with biliary atresia, which did not allow sufficient time to assess exposure to acute insults specifically from infection with hepatotropic viruses. The gradual progressive nature of liver dysfunction in biliary atresia also meant that majority of these children with chronic, progressive cholestasis and decompensation would be excluded from APASL definition. By contrast, the European and North American criteria provide broader definitions of acute triggers of decompensation that include intra and extrahepatic events such as bacterial infections. Studies using these criteria may include a wider variety of liver disorders, including biliary atresia. Moreover, geographic factors may influence the prevalence of specific liver disorders and hepatotropic viruses. Nonetheless, until a standardised criteria is used across studies, it will be impossible to draw any conclusion on aetiologic factors associated with paediatric ACLF.
PRECIPITATING TRIGGERS
Data on the precipitating triggers of ACLF in children are mostly from studies from India using the APASL criteria. Superimposed viral hepatitis (majority hepatitis A and E viruses) accounted for 22–81% of acute insults leading to ACLF, while poor control of underlying autoimmune liver disease or Wilson’s disease (13–48%) and drug induced liver injury (6–11%) were other common triggers.10-12,18,25,26 Although, the APASL definition includes only hepatic insults as a cause of ACLF, Jagadisan et al.25 reported that the concomitant presence of bacterial sepsis with hepatotropic virus in 41% of children with ACLF (7 out of 17) was associated with a higher mortality rate of 71%, as compared to 59% without bacterial sepsis. Compared to 20 children with biliary atresia from the UK described by D’Souza et al.19 using the EASL-CLIF criteria, ACLF was precipitated by sepsis (45%) and gastrointestinal bleeding (40%). Similarly, gastrointestinal bleeding (30%) was found to be an important triggering event leading to decompensation in North American children with ACLF from Banc-Husu et al.’s study.14 It is noteworthy that distinct geographic backgrounds of the different populations that were studied do play a significant role, as hepatitis A and E are highly endemic in India, but are not as prevalent in Western countries.
In adults, the main triggers are bacterial infections, relapse of hepatitis B, active alcoholism, and gastrointestinal bleeding.21,27 Interestingly in 20–50% of cases of adult ACLF, the trigger remains unknown,21,28 and this is similarly reflected in a paediatric study where no cause was found in 23% of ACLF.10
OUTCOME AND PROGNOSTIC SCORES
Across all definitions and population groups, mortality rates for both paediatric and adult ACLF are universally high, emphasising the need for early recognition and expedited treatment of ACLF. The overall mortality rate without transplantation in children is 25% at 28 days, rising to 30–50% within 90 days,12,24 which are relatively consistent with mortality rates derived from adult studies that quote a mortality rate of 25–40% at 28 days6,22,27 and 40% at 90 days.22
In adult studies, the CLIF-C OF score has been shown to have higher predictive accuracy than model for end stage liver disease in predicting survival.29 The score takes into account the number of organ failures, and incorporates age and white cell count to calculate an ACLF score with a predicted mortality rate. However, this has not been validated for use in children. For children, a paediatric adaptation of the chronic liver failure sequential organ failure assessment (pCLIF-SOFA) score has been created that scores six impairments (respiratory, neurologic, circulatory, haematological, renal, and liver) based on paediatric-appropriate cut-offs (Table 2). A pCLIF-SOFA score of ≥11 identified 28-day mortality with a sensitivity of 94.9% and specificity of 91.5%.13 When comparing the pCLIF-SOFA to the paediatric end stage liver disease (PELD) score, both pCLIF-SOFA and PELD scores at cut-off values >8 and >30 respectively on admission predicted death in children with acute liver failure (ALF) with high sensitivity, with pCLIF-SOFA demonstrating superior specificity, positive predictive value, and negative predictive value as compared to PELD.30 Claude et al. also showed that a pCLIF-SOFA score of >9 was predictive of mortality within 28 days with a sensitivity of 87.8% and a specificity of 77.3%, while a pCLIF-SOFA score of >7 was associated with increased odds of liver transplantation on day-60.20
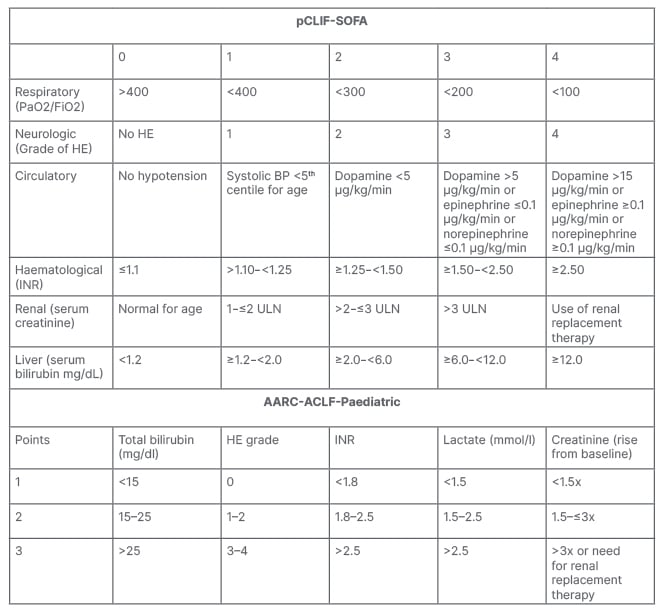
Table 2: Paediatric adaptations of the chronic liver failure sequential organ failure assessment (pCLIF- SOFA) score and Asian Pacific Association for the Study of the Liver (APASL) acute-on-chronic liver failure (ACLF) Research Consortium (AARC) acute on chronic liver failure (AARC-ACLF-Paediatric) score.
HE: hepatic encephalopathy; INR: international normalised ratio; ULN: upper limit of normal.
Lal et al.24 evaluated the APASL ACLF Research Consortium (AARC) acute-on-chronic liver failure (AARC-ACLF) score and its paediatric-adapted version (Table 2) in prognosticating ACLF in children. The authors found that AARC-ACLF and CLIF-SOFA scores were superior to other prognostic scores in paediatric ACLF, and paediatric modifications of AARC-ACLF and CLIF-SOFA did not perform better than their original scores, all having AUROC of greater than 0.9 for predicting poor outcome in paediatric ACLF. A cut-off of 11 or more in these scores, and/or an increasing score at Day 4, were found to be predictive of death or liver transplantation.
Table 3 summarises and compares the differences between paediatric and adult ACLF.
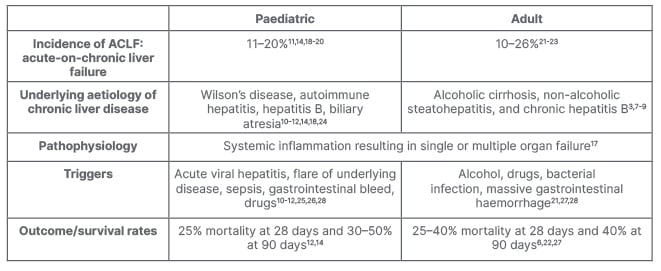
Table 3: Comparison between paediatric and adult acute-on-chronic liver failure.
MANAGEMENT
The mainstay of treatment is early diagnosis of ACLF to treat the precipitating event and then provide supportive therapy to hepatic and extrahepatic complications. Whilst there are several therapeutic options to help delay progression of ACLF, the only definitive life-saving therapeutic option is liver transplantation.
Based on paediatric studies,10,18,25 suggested investigations to identify the trigger such as bacterial cultures from blood, urine, and stool; testing for hepatotropic viruses such as hepatitis A–E, cytomegalovirus, Epstein–Barr virus, herpes simplex virus, parvovirus, human herpes virus-6 and enterovirus; fungal studies; and toxicology studies should be considered. For the underlying cause of liver cirrhosis if not yet diagnosed, screening for autoimmune hepatitis, Wilson’s disease, as well as other metabolic liver disorders may be performed. Hepatobiliary imaging such as with ultrasonography may confirm features related to cirrhosis and portal hypertension, and also allows objective assessment of ascites. To assess hepatic function, the prothrombin time, international normalised ratio, serum glucose, ammonia, lactate, bilirubin, albumin, ammonia, and liver transaminases should be checked. Initial antimicrobial coverage for patients with ACLF should include broad spectrum antibiotics and antifungals.
Depending on the complications of liver failure, supportive treatment would include fluid and electrolyte management, intravenous Vitamin K supplementation, fresh frozen plasma and/or platelet infusion for active clinical bleeding, albumin replacement, diuretics and/or paracentesis for ascites, vasoactive drug therapy and/or gastroscopy for variceal bleeding, neuroprotective measures for encephalopathy, dextrose infusions for hypoglycaemia, and/or dialysis for hepatorenal syndrome.
In view of the high short-term mortality rate, it is suggested that patients who show no clinical improvement at 3–7 days after ACLF is diagnosed should be considered for emergency liver transplantation (LT).31 The majority of patients achieved their final grade of ACLF within the first week. A paper published by the APASL ACLF Research Consortium32 also proposed a 7-day threshold, or the ‘golden window’ for organ support, or prioritisation for definitive organ transplant prior to onset of sepsis to improve survival.
Extracorporeal liver support systems (ECLS) are used to prolong the window of opportunity while awaiting LT in both adults and children with liver failure. An effective ECLS system should perform three primary hepatic functions: detoxify, stimulate liver regeneration, and prevent further injury to the liver.33 Numerous systems have been investigated, but no system has demonstrated improvement to mortality in ACLF in prospective trials. Molecular adsorbent recirculating system has shown significant improvement to serum bilirubin and creatinine levels within 4 days, and reduction in the proportion of patients with clinically significant hepatic encephalopathy.34
Granulocyte colony-stimulating factor (GCSF) has shown promising results in improving survival rate and prognosis of patients with liver failure.35 GCSF has been reported to reduce the rate of complications such as sepsis and multiorgan failure, possibly from improved neutrophil function and immunomodulation.36 The mechanism of GCSF in the treatment of ACLF remains unclear, but has been proposed to involve the promotion of hepatocyte regeneration and facilitation of migration, proliferation, and differentiation of stem cells into the damaged liver. A meta-analysis of six clinical studies by Huang et al.35 suggested that GCSF may optimise the efficacy of medical treatment or ECLS in patients with ACLF, hence preventing progression of multiple complications and multiorgan failure. However, a multicentre, prospective, open-label Phase II study37 that was published just after the aforementioned meta-analysis, reported no significant benefit in patients with ACLF versus standard medical therapy alone. The 90-day transplant-free survival rate in the GCSF patient group was 34.1% compared with 37.5% in the standard medical therapy group. Transplant-free and overall survival rates, liver function scores, and infection rates at 360 days did not differ between the two groups. The aforementioned studies were mainly conducted in adult patients, but a study investigating the benefits of GCSF in paediatric ACLF patients also reported ineffectiveness in improving the survival outcome on Days 30 and 60 of therapy.38
LT remains the only definitive therapeutic option in patients who do not recover from ACLF. Numerous studies have shown good 1-year survival rates (81–92%) in ACLF patients post-LT.39-41 Identification of patients with ACLF who would benefit from early liver transplantation and organ allocation remains unclear in both the adult and paediatric populations.42,43
CONCLUSION
ACLF is increasingly recognised as a clinical syndrome of critical importance in patients with chronic liver disease and cirrhosis associated with significant mortality. Current paediatric literature remains scarce, and study groups have used differing inclusion criteria that have limited generalisability of data (Table 4).
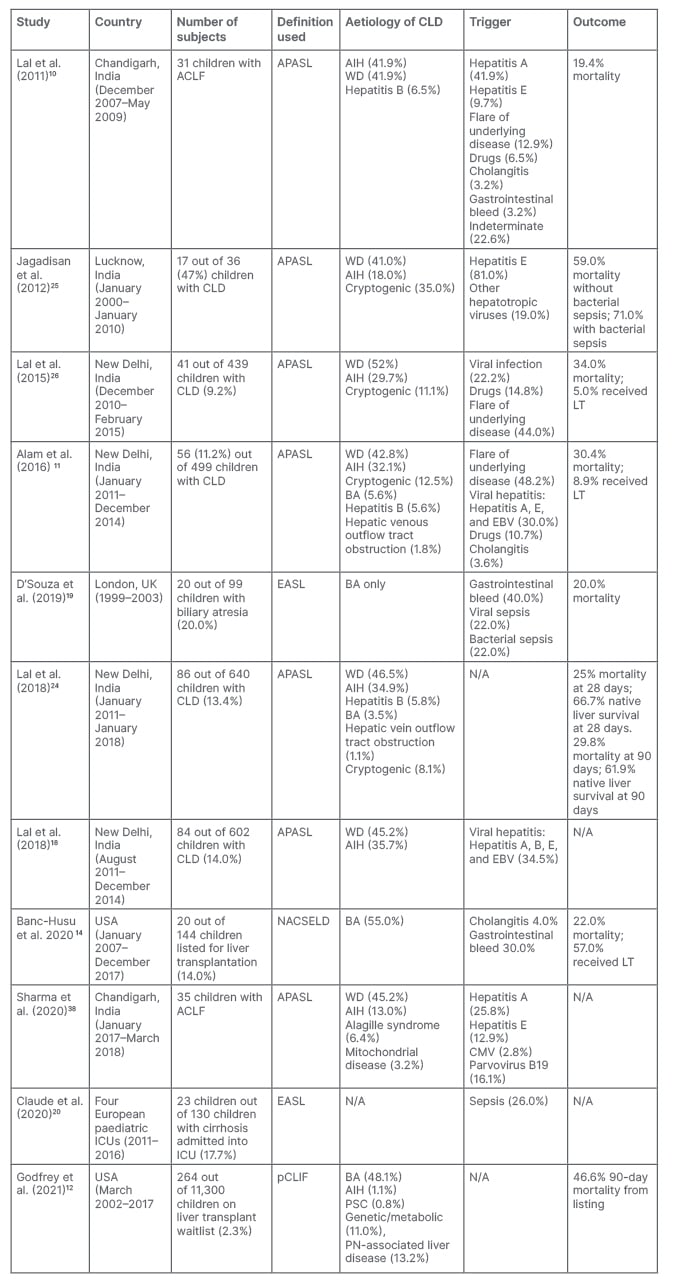
Table 4: Summary of relevant paediatric studies on acute-on-chronic liver failure.
ACLF: acute-on-chronic liver failure; AIH: autoimmune hepatitis; APASL: Asian Pacific Association for the Study of the Liver; BA: biliary atresia; CLD: chronic liver disease; CMV: cytomegalovirus; EASL: European Association for the Study of the Liver; EBV: Epstein–Barr virus; LT: liver transplantation; N/A: not applicable; NASCELD: North American Consortium for the Study of End Stage Liver Disease; pCLIF: paediatric chronic liver failure; PN: parenteral nutrition; PSC: primary sclerosing cholangitis; WD: Wilson’s disease.
There is a pressing need for international paediatric groups and societies to develop and validate the diagnostic criteria for paediatric ACLF, so that further collaborative research may provide greater understanding on the epidemiology, pathophysiology, risk factors, and outcome of this clinical entity.







