Abstract
Antibiotic resistance in patients with cirrhosis continues to draw significant attention. With a propensity to frequent hospitalisations, patients with cirrhosis are subject to frequent antibiotic prescription. This increases their risk of developing resistance to one or more antimicrobial agents, making management of their condition particularly challenging. Despite advancements being made in the management of liver disease, mortality rates continue to rise: almost 5-fold in those <65 years of age while remaining the leading cause of death in those 35–49 years of age. Alternative therapeutic options to prevent disease progression and cirrhosis-associated complications are urgently required; rifaximin is one such example. The medication use in patients with cirrhosis demonstrates additional benefits beyond current licensed use in the UK, that being for the prevention of hepatic encephalopathy and traveller’s diarrhoea; rifaximin has especially been explored beyond current licensed use in the context of enteric-driven pathologies. Through the therapy’s key central action as a broad-spectrum antimicrobial, rifaximin has the ability to modulate the gut–liver axis via removal of gut microbial products associated with the progression of cirrhosis and its sequalae.
The benefits of rifaximin use continues to gather momentum, given its non-absorbable nature and well-tolerated side-effect profile, and these require consideration. With broad-spectrum antimicrobial properties, its use may assist in overcoming the conundrum posed of antibiotic resistance amongst patients with cirrhosis. This literature review discusses the chemical and antimicrobial properties of rifaximin, its licenced indication for use, and its reported benefits beyond this, as well as concerns regarding rifaximin resistance.
INTRODUCTION
Liver disease constitutes a major global health burden, with mortality rates increasing 4-fold since the 1970s and 5-fold in those <65 years of age. Liver disease represents the leading cause of death in those 35–49 years of age, and the third most common cause of death in those 50–64 years of age in 2019.1 Its rising incidence and rate of prevalence are largely attributable to risk factors for the most common conditions, including growing levels of alcohol consumption, the obesity epidemic, and viral hepatitis.2,3
Cirrhosis remains the primary driver of liver-related deaths. Pathologically, progression from inflammation to fibrosis and on to cirrhosis occurs as a result of continual exposure to a hepatic insult.4 Treating the underlying aetiology of liver disease can slow progression, although in some cases the only cure remains liver transplantation; however, this is restricted to selected patients only and is further limited by organ supply.5 This highlights the urgent need to explore novel therapeutic options that ameliorate disease progression and its complications.
As a result of progressive immune dysfunction, patients with cirrhosis are at risk of recurrent bacterial infections, which are recognised as a major cause for the development of liver-related complications, advancement of liver failure, and repeated hospitalisations.6 These patients are frequently prescribed antibiotics as treatment or for infection prophylaxis, making them susceptible to developing multiple drug-resistant and extensively drug-resistant organisms, for which limited therapeutic options exist.7,8 This review discusses the growing understanding of the possible role of rifaximin as an agent that may impact upon the trajectory of chronic liver disease, as a tool in the prevention of antibiotic resistance in chronic liver disease, and other potential areas of clinical benefit.
RIFAXIMIN
The use of rifaximin beyond its current licensed indications has been explored, especially in the context of enteric-driven pathologies. Translation into clinical practice has been hindered by a paucity of robust clinical trials. A considered review of the current understanding and uncertainties surrounding rifaximin use in decompensated cirrhosis is needed to inform appropriate future trial design, and therefore is discussed further here.
Chemical Structure
Rifaximin is a semi-synthetic, non-aminoglycoside oral antibiotic derived from rifamycin.9 Its pyridoimidazole ring differentiates it from other rifamycin derivates and leaves rifaximin water-insoluble, therefore inhibiting absorption in the gastrointestinal tract.10,11 Its molecular mass size of 789.9 Da enables self-association, further preventing systemic absorption.12 Consequently, adjustments of dosing are not required.13 Following oral administration, the concentration of detectable rifaximin in blood and urine is less than 0.4%, but up to 97.0% in stool, suggesting that its bioavailability is localised to the gastrointestinal tract.14
Antimicrobial Properties
Rifaximin inhibits bacterial RNA synthesis through its action on the β-subunit of bacterial DNA-dependent RNA polymerase.15In vitro studies have demonstrated that rifaximin has antibiotic coverage against Gram-positive and Gram-negative aerobic and anaerobic bacteria (Table 1). It also shows effectiveness against the protozoa Cyrptosporidium parvuum and Blastocystis hominis.17 Rifaximin has demonstrated intermediate in vitro activity against various enteropathogens, with minimum inhibitory concentrations (MIC)50 and MIC90 values ranging 8–64 and 16–128 μg/ mL, respectively.18,19 Yet, rifaximin achieves potent in vivo activity against enteropathogens, which can be explained by its extremely high luminal concentrations (4,000–8,000 μg/g) that far exceed MIC90 values.18,20 Certain enteric bacteria, such as Clostridium difficile, have shown increased susceptibility to rifaximin with lower MIC50 (≤0.25 μg/mL).16,18
The solubility of rifaximin is 70–120 times greater in bile than in aqueous solution. This aids action in the small intestine which is bile-rich, compared to the aqueous colonic environment.21 Encouragingly, rifaximin does not significantly alter the composition of the gut microbiota but rather selectively targets bacteria ( Table 1 ).
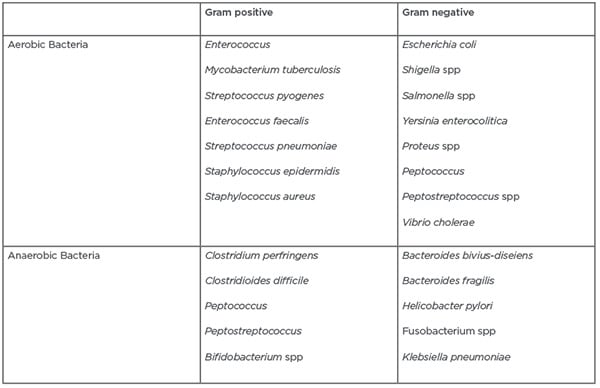
Table 1: Isolated bacteria for which rifaximin has anti-microbial activity.13,16
Concurrently, rifaximin favours the growth of beneficial bacterial species.22
Indications for Rifaximin Use
In view of its enterically localised bioavailability, rifaximin has primarily complemented the management of gastrointestinal pathology: travellers’ diarrhoea, infectious diarrhoea, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome, diverticular disease, infection prophylaxis post-colorectal surgery, and inflammatory bowel disease.23 Over the last decade it has also emerged as a key therapeutic agent in the management of hepatic encephalopathy (HE).24 Despite widespread use in Europe for the above conditions,25 rifaximin is licensed only to minimise recurrent episodes of overt HE (high-quality evidence, strong recommendation)26 and to treat travellers’ diarrhoea in the UK and USA (high-quality evidence, strong recommendation),27 as well as to manage irritable bowel syndrome with diarrhoea in the USA (moderate-quality evidence, conditional recommendation).28-30
Hepatic encephalopathy
Rifaximin improves the severity of acute and chronic HE by targeting deaminating enteric bacteria producing systemically absorbed nitrogenous compounds, including ammonia.31 Concurrently, rifaximin reduces pathogenic bacterial species, thereby reconditioning the gut microbiome and reducing systemic endotoxaemia.32
The beneficial effects of rifaximin in the treatment of overt HE were initially established in a double-blind, placebo-controlled trial by Bass et al.,24 demonstrating that rifaximin was associated with a 6-month risk reduction in episodes of overt HE and HE-associated hospitalisations.24 In a further randomised controlled trial (RCT), rifaximin plus lactulose demonstrated superiority over lactulose alone in decreasing hospital stay and all-cause mortality in patients with decompensated cirrhosis (23.8% versus 49.1%; p<0.05).33 These results were reinforced by a meta-analysis of 19 RCTs including 1,370 patients.34 Rifaximin use for HE in clinical practice is further advocated by its short- and long-term safety profiles. Its tolerance is well acknowledged, especially when compared to alternative treatment options.35,36 Furthermore, its demonstrated benefits are beyond the physical alone, and it significantly improves health-related quality of life of patients with cirrhosis with HE.37 In light of this, rifaximin is recommended in national and international consensus guidelines as an effective adjunct to lactulose for the prevention of recurrent overt HE.38
Other Potential Benefits of Rifaximin
Rifaximin has been shown to effect bacterial translocation, thereby modulating the gut microbiome. Therefore, it has the potential to modify the interplay of the gut–liver axis, central to progression of cirrhosis and its sequelae.39 Data to support this have been reported, though changes to guidelines are yet to be implemented.
Spontaneous bacterial peritonitis
With guidelines recommending the use of norfloxacin for the management of spontaneous bacterial peritonitis (SBP), Campillo et al.40 described the effects of long-term antibiotic administration, namely increased severity of hospital-acquired, Gram-positive staphylococcal infections and quinolone resistance. Presently, antibiotic resistance poses a significant challenge in the management of infection in patients with cirrhosis, with a pressing need for alternative prophylactic antibiotics.
Organisms responsible for the development of SBP are both Gram-negative and Gram-positive. Frequently isolated Gram-negative pathogens include Escherichia coli, Klebsiella pneumoniae, and Streptococcus pneumoniae.41 Gram-positive pathogens implicated include methicillin-resistant Staphylococcus aureus and enterococci.42 As shown in Table 1, rifaximin demonstrated antimicrobial effectiveness against all these pathobionts and so its use in preventing SBP has been previously investigated.
In an RCT comparing rifaximin to norfloxacin in 262 patients affected by cirrhosis, rifaximin was superior in reducing the incidence of recurrent SBP over 6 months (3.88% versus 14.13%; p=0.04).43 Similar findings were established for the primary prevention of SBP in patients with hepatitis-C-induced cirrhosis.44 In a meta-analysis comparing rifaximin to various systemic antibiotics, rifaximin lowered SBP risk by 47% compared to placebo for primary prevention, and by 74% compared to systemic antibiotics for secondary prevention.45 Prospective open-label studies have been less optimistic, demonstrating mixed results that concluded rifaximin did not consistently or reliably prevent SBP.46 Very few studies have assessed secondary SBP occurrence in patients with cirrhosis, concomitantly prescribed antibiotic prophylaxis for SBP and rifaximin for HE. Consequently, rifaximin alone is not recommended for the treatment or prevention of SBP as per consensus guidelines.8
Endotoxaemia and haemodynamics
Endotoxins IL-6 and TNF-α have been shown to be associated with development of cirrhosis-associated immune dysfunction, hyperdynamic circulation, and multi-organ dysfunction. These endotoxins can potentially be influenced by rifaximin use. Kalambokis et al.47 demonstrated in 13 patients with advanced-disease cirrhosis that rifaximin use reduced IL-6 and TNF-α, subsequently improving systemic haemodynamics, cardiac output, and plasma renin activity.47 Furthermore, 28 days of rifaximin administration was shown to significantly lower plasma endotoxin levels and hepatovenous pressure gradient measurements, independent of β-blocker use.48 After 5-year follow-up of the same cohort, the rifaximin-treated cohort demonstrated reduced complication rates of variceal haemorrhage, hepatorenal syndrome, and SBP, as well as survival benefit compared to the control group.49 Rifaximin modulates gut epithelial physiology and prevents bacterial adherence and internalisation, reducing the release of pro-inflammatory cytokines (IFN-γ, IL-4, IL-6, IL-8, IL-12, and VCAM-1) implicated in cirrhosis-associated immune dysfunction.50 Partly responsible for this phenomenon is the rifaximin-induced activation of the gut-specific pregnane-X receptor,18 thereby suppressing NF-κB signalling, a pathway responsible for pro-inflammatory cytokine release.51 This may help to reduce intestinal permeability, a recognised process contributing to infections in cirrhosis.18
Small intestinal bacterial overgrowth
The role of SIBO in bacterial infection in cirrhosis is well recognised.6 Treatment for this condition is largely based on antibiotic therapy, for which rifaximin is one efficacious option.52 In a meta-analysis of 32 studies and 1,331 patients, rifaximin treated SIBO in 70.8% of patients (95% confidence interval: 61.4–78.2).53 SIBO occurs frequently in cirrhosis, but few studies have interrogated antibiotic effectiveness in this cohort. One small-scale study by Zhang et al.54 reported that rifaximin was clinically effective in treating SIBO in 76% of patients with a confirmed diagnosis of cirrhosis.
Cirrhosis-associated morbidity
Recent evidence has proposed that rifaximin use may reduce complications associated with cirrhosis. In a Phase III RCT, 6-month-long rifaximin administration reduced the relative risk of a first cirrhosis-associated complication by 59% in patients with advanced disease.55 In a retrospective analysis of 101 patients awaiting liver transplantation, rifaximin increased the time interval to readmission and reduced incidence of portal hypertensive complications: variceal bleeding, complications of ascites, and hepatorenal syndrome. Patients receiving rifaximin exhibited a survival benefit and were less likely to be prioritised for organ transplantation, highlighting the benefits of rifaximin.56,57
In addition to clinical benefits, rifaximin therapy may have wider socio-economic advantages, especially in limited resource settings. In a UK-based retrospective study, rifaximin significantly reduced 30-day readmissions and emergency department attendances, as well as hospital and critical care bed days, 6 and 12 months post-commencement of treatment,58 incurring annual savings of 1,480–3,228 GBP per patient.59
RIFAXIMIN RESISTANCE
Until recently, resistance to rifaximin was thought to be uncommon.60 This perception changed when a mutation was identified in the rpoβ gene. This encodes the target site of rifaximin, giving rise to concerns regarding the emergence of resistance in patients with cirrhosis on long-term rifaximin treatment.13,61 These concerns were substantiated by an observational study of 388 patients with cirrhosis, of whom 46 (11.9%) developed C. difficile infection. Importantly, 30.4% of those who developed C. difficile infection were established on rifaximin for HE prophylaxis. Overall, C. difficile resistance to rifaximin was observed in 34.1% of cases and in 84.6% of patients who had previously received rifaximin. These findings stand in contrast to previous clinical studies and in vitro data, in which rifaximin demonstrated good antibacterial activity against C. difficile and in which the emergence of resistant clones was rare.62 With resistance patterns being considerable in those with previous and no previous exposure, rifaximin resistance may be an emerging problem in cirrhosis and will require further investigation.
CONCLUSION
While it is currently licensed for the treatment of recurrent episodes of HE, the full therapeutic potential of rifaximin in liver disease may be underutilised. Rifaximin displays broad-spectrum antimicrobial activity against pathobiont enteric bacteria and demonstrates anti-inflammatory properties. It also has the ability to modulate the gut microbiome, thereby preventing bacterial translocation.18 These processes are fundamental in the pathogenesis of cirrhosis-associated infection and therefore rifaximin use may be effective in this scenario.6 However, there remains a lack of robust evidence in the form of adequately powered RCTs to translate these perceived benefits into clinical practice. To date, studies have focused only on the prevention of SBP, rather than all infections. There remains uncertainty regarding rifaximin resistance; yet, based on the current evidence, rifaximin use beyond the licensed indications may be a significant step forward in the management and prevention of cirrhosis-associated morbidity and mortality.








