Abstract
The Liver Meeting 2016, held by the American Association for the Study of Liver Diseases (AASLD) in Boston, Massachusetts, USA, supported the presentation of both recent clinical research and basic research in the area of liver disease from renowned experts to target the diverse needs of hepatology professionals. Posters presented on hepatitis B and oral presentations discussing chronic hepatitis infections are summarised within this article.
VIRAL HEPATITIS B POSTER SESSION
Hepatitis B virus (HBV) is one of the most common causes of cirrhosis and hepatocellular carcinoma (HCC), with around 25% of individuals with chronic hepatitis B (CHB) infection developing serious liver disease.1 Outcomes for CHB infection have improved with the introduction of new antiviral therapies and the improved management of patients; HBsAg+ patients may now be considered for pre-transplant, prophylactic post-transplant, and therapeutic post-transplant treatment approaches.1 The Viral Hepatitis and Orthotopic Liver Transplantation poster session at the AASLD 2016 Liver Meeting covered important topics related to HBV screening, therapy and resistance, viral variance, host responses, and risk factors.
Low Rates of Hepatitis B Virus (HBV) Screening and Low Rates of HBV Awareness Among High-Risk Patients at a Large, Urban, Safety-Net Hospital
Early detection and treatment of HBV infection can reduce the likelihood of liver disease progression and development of HCC. The lack of awareness of HBV amongst patients and healthcare providers contributes to suboptimal screening, which is particularly important for high-risk populations where delayed diagnosis can lead to more advanced disease and increased mortality.
Dr Robert J. Wong,2 Alameda Health System, Department of Internal Medicine, Highland Hospital, Oakland, California, USA, presented findings from a prospective study of adult patients attending outpatient endoscopy from July 2015–March 2016 at a large, ethnically diverse safety-net hospital. Patients were evaluated for risk-based HBV screening using US Preventive Services Task Force (USPSTF) guidelines.3 Appropriate screening and awareness of HBV results among high-risk patients were stratified by patient demographics and evaluated by multivariate logistic regression models. In total, 869 patients were screened for HBV risk factors, of which 62% (n=535) were high-risk for chronic HBV (50% male, 88% foreign-born, 18% had a history of incarceration, and 12% had human immunodeficiency virus [HIV] co-infection). Within the high-risk cohort, 25% (n=81) had received prior testing, of which 23% (n=16) were aware of past results (HBV+ patients were more likely than HBV− patients to be aware of prior results; 75% versus 19%, p=0.01). Among high-risk patients without prior testing, 96% accepted testing; however, those with concomitant HIV infection were less likely to accept testing compared with HIV− patients (67% versus 97%, p=0.03). In total, 446 patients accepted HBV testing. Among these, 70% completed testing and 2.3% (n=7) were HBsAg+. HBV DNA testing was completed by five patients, all of whom were positive for HBV DNA and were linked to a HBV clinic.
Findings showed that the majority of patients were at a high risk of CHB and amongst those identified, only 25% had received prior HBV testing. Although nearly 70% of patients accepted and completed testing, CHB prevalence was 2.3% and only 60% of these patients were linked to a HBV clinic, thus targeted education and outreach of the importance of screening and linkage of care is necessary for patients and healthcare providers.
Progression from Immune Tolerant to HBeAg-Negative Hepatitis B is Associated with Increased Virus Sequence Diversity and Prevalence of BCP and Precore Variants
CHB natural history is characterised by evolution through multiple phases of host–virus interplay that results in the selection of HBV variants that are defective for HBeAg production. Currently, progression from the early immune tolerance (IT) to immune clearance (IC) phase, before late immune reactivation (IR), is suggested. Recently, it has been shown that frequency of basal core promoter (BCP) and precore (PC) HBV variants, and HBV sequence nucleotide diversity, are associated with clinical phenotype and treatment outcomes.
Prof Peter A. Revill,4 Victorian Infectious Diseases Reference Laboratory (VIDRL), Melbourne, Australia, presented the results of a cross-sectional analysis of the virological phenotypes determined from IT, IC, and IR-phase patients with CHB from three large, randomised controlled studies. The analysis was grouped into patient cohorts based on phase: IT, IC, and IR. The IT cohort evaluated tenofovir disoproxil fumarate (TDF) versus TDF plus emtricitabine treatment (HBeAg+, HBV DNA >7.28 log10 IU/mL, alanine aminotransferase [ALT] <1x upper limit of normal [ULN]), the IC cohort evaluated TDF in IC patients (HBeAg+, HBV DNA >6 log10 copies/mL, ALT >2x ULN but <10x ULN), and the IR cohort evaluated TDF in IR patients (HBeAg−, HBV DNA >5 log10 copies/mL, ALT >1x ULN but <10x ULN).
Data from 370 patients were included (IT: n=98; IC: n=158; IR: n=114). Baseline characteristics varied between groups (Table 1). HBsAg, HBV DNA, and HBeAg levels differed by phase of disease and were consistent with the natural history of CHB (Table 1). As the disease phase progresses, the dominant genotype changes from B and C, to D; in the IT group genotypes B and C were most common, in the IC group all genotypes were present, and finally, in the IR group, genotype D was most prevalent.
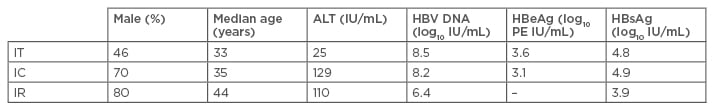
Table 1: Baseline characteristics of patients in each phase of disease.
IU: international units; ALT: alanine transaminase; HBV DNA: hepatitis B virus deoxyribonucleic acid; HBeAg: hepatitis B e antigen; PE: Paul Ehrlich units; HBsAg: hepatitis B surface antigen; IC: immune clearance; IR: immune reactivation; IT: immune tolerance.
BCP (A1762T and G1764A) and PC (G1896A) variants were detected in all CHB phases and the proportion of wild-type (WT) variants decreased significantly as disease progressed (IT: 76%, IC: 31%, and IR: 3%; p<0.0001). BCP-only and PC-only variants were detected in higher proportions in the IC phase (IC BCP-only: 27%; IC PC-only: 27%), whereas the highest proportion of BCP+PC variants was detected in the IR phase (55%, p<0.0001). BCP and PC variant frequencies were compared across genotypes within CHB phases; in cases with BCPA1762T variants (±G1764A ±PCG1896A), genotype B cases with BCP variants were significantly lower (p<0.05) in all disease phases compared with genotype C cases, and genotypes A and D cases with BCP variants showed no significant difference in frequency (median frequencies were >94% in both genotypes). In cases with PCG1896A variants (±BCPA1762T ±BCPG1764A), genotype B cases with PC variants were significantly higher in IC and IR-phase patients (p<0.05) compared with genotypes A, C, and D, and the frequency of PC variants in the IR-phase was significantly higher in genotype D (p=0.0446) than genotypes A and C. HBV sequence diversity across the full-length HBV genome increased as disease progressed from IT through IC to IR.
The accumulation of clinically relevant variants in the BCP and PC regions, as well as increased HBV sequence nucleotide diversity, across the full length of the HBV genome, is characteristic of progression from the IT to IC, to IR-phase disease.
Characterising Immune Recovery by Antibody Response, and the Association with a HBsAg Epitope Profile Predictive of HBsAg Clearance in a TDF Therapy Cohort of Chronic Hepatitis B (CHB) Patients Who Achieved Functional Cure
One of the primary treatment goals for CHB is HBsAg clearance. Unfortunately, few reliable predictive biomarkers exist to accurately identify patients who are most likely to achieve this rare outcome.
Dr Renae Walsh5 from the VIDRL, Melbourne, Australia, presented findings from an analysis of a Phase III study (GS-US-174-0103 [G103])6 that used epitope mapping of TDF-treated patients with CHB to identify a clearance profile (CP) genotype associated with HBsAg loss/seroconversion.7,8 The findings presented at the AASLD meeting characterised the relationship between the HBsAg CP and coexisting antibodies to HBsAg (anti-HBs)/HBsAg complex, with HBsAg decline, loss, and seroconversion.
To this end, 181 samples from 25 patients, who were HBeAg+ prior to treatment and eventually developed an HBsAg CP, were serologically measured for HBsAg, HBeAg, ALT, and ‘free’ anti-HBs levels. A significant association between HBsAg CP and an outcome of HBsAg loss/seroconversion was demonstrated prior to HBsAg loss at Week 48 (p=0.015) and by the end of the study (Week 192) or the last time point prior to HBsAg loss (p=0.001). The development of an HBsAg CP with an outcome of HBsAg loss also significantly correlated to the level of HBsAg decline and an ALT flare. Complexed anti-HBs, when correlated with HBsAg CP, was significantly associated with HBsAg loss (p=0.004) and indicates development of an immune response.
Findings from this analysis suggest that an antibody response may be used as a predictive biomarker in patients with an HBsAg CP to provide information regarding those more likely to obtain HBsAg loss and functional cure.
Characterization of Intrahepatic Viral Antigens and Immune Markers in Chronic Hepatitis B Patients by Multiplex Immunofluorescence
The characterisation of HBV surface and core antigens by immunohistochemistry (IHC) is useful for demonstrating the range of viral expression in hepatocytes, but offers little information about host responses. Additionally, the impact of CHB infection on the immune microenvironment in the liver is poorly understood.
Dorothy French,9 Gilead Sciences, Inc., California, USA, presented the findings of a study that aimed to assess viral antigens by IHC, characterise types and frequency of immune cells in CHB biopsies, and determine the spatial relationships between viral antigens and immune-cell populations. Tissue samples included 48 baseline liver biopsies from patients with CHB infection in an open-label Phase IV trial of TDF + pegylated interferon peg-IFN and 13 biopsies representing a range of HBsAg and hepatitis B core antigen (HBcAg) expressions.
Results showed that mean distributions of HBsAg+ and HBcAg+ pathologist scores for HBV biopsies were 37% and 18%, respectively, and that HBsAg and HBcAg expression was often discordant within the same biopsy; although, for a subset of samples, positively-stained hepatocytes for HBsAg and HBcAg were mutually exclusive. Where there was mutual exclusivity, CD8+ T cells were less abundant in HBsAg+ regions and CD8+ cells showed a tendency to cluster around HBcAg+ hepatocytes. CD8+ T cells within proximity to HBsAg+ hepatocytes were more common versus HBcAg– hepatocytes (4.0 versus 2.2, p<0.001). CD8+ cells were mostly programmed cell death protein 1 (PD-1)+, especially in areas of HBcAg+ hepatocytes, and PD-L1+/CD68+ macrophages tended to aggregate in HBcAg+ regions. In contrast, CD20+ cells were rare.
Liver biopsies from patients with CHB infection were successfully processed using immunofluorescence to assess markers of viral activity and host immune response; there was a wide range of HBsAg and HBcAg expression between patients, and many CD8+ cells were less common in HBsAg+ regions and had a tendency to cluster around HBsAg+ hepatocytes. PD-L1+/CD68+ macrophages co-localised with CD8+/PD-1+ T cells and CD20+ B cells mostly resided within the periportal regions.
The Hepatitis B Virus X Gene is Transcribed Very Early After Infection of Primary Human Hepatocytes and Prior to Expression of the Other HBV Genes
It has previously been proposed that the HBV X protein (HBx) promotes the degradation of Smc5/6 to prevent the silencing of covalently closed circular DNA (cccDNA).10,11 However, this model raises a paradox: how is HBx expressed if it is required to alleviate transcriptional suppression of cccDNA by Smc5/6? Three possible explanations include: that HBx or HB core mRNA is packaged within HBV particles, viral-like particles, or microvesicles; HBx is transcribed early after infection from newly formed cccDNA prior to restriction by Smc5/6; or that Smc5/6 inhibits transcription of all HBV RNAs except HBx.
Dr Rudolf Beran,12 Gilead Sciences, Inc., San Francisco, California, USA, presented the findings of a study that aimed to characterise the viral kinetics within primary human hepatocytes (PHH) infected with WT HBV and HBx– HBV (HBV∆X). At Day 13, it was found that both WT HBV and HBV∆X can infect PHH. Most cells expressed the HBx and HBV core by Day 2 and Day 4 post-infection, respectively. The transcription of all HBV mRNAs was suppressed by Smc5/6 in the absence of functional HBx. Within 4 hours post-infection, HBx RNA was detected and low levels of cccDNA were detected by 24 hours post-infection with WT HBV and HBV∆X. Substantial cccDNA transcription and HBeAg production was seen by Day 4 post-infection.
HBx RNA was detected very early after infection and prior to Smc6 degradation and transcription of other HBV genes. Smc5/6 inhibited transcription of HBx, as well as other HBV genes. As a whole, these data suggest that early expression of HBx is a strategy to counteract Smc5/6 restriction of cccDNA and future studies should focus on determining the nature and origin of HBx RNA.
No Resistance to Tenofovir Alafenamide Detected Through 48 Weeks of Treatment in Patients with Chronic Hepatitis B
Tenofovir alafenamide fumarate (TAF), a new tenofovir (TFV) prodrug, has greater plasma stability than TDF, enhances delivery of active drug to hepatocytes,13-15 and has reduced circulating levels relative to TDF.16,17 Mutations that confer resistance against polymerase/reverse transcriptase have been identified in HBV against lamivudine, adefovir, telbivudine, and entecavir (ETV); however, no evidence of resistance has been documented against TDF with up to 8 years of treatment.18,19
Dr Henry Lik-Yuen Chan,20 Faculty of Medicine, The Chinese University of Hong Kong, Hong Kong, presented the results of two Phase III randomised trials of patients with CHB who qualified for resistance surveillance on TAF treatment for 48 weeks. Known resistance mutations to nucleos(t)ide analogues (NUCs) at baseline and amino acid substitutions within pol/RT were detected and identified, and analysis of whether the substitutions alter susceptibility to TFV was carried out using in vitro phenotypic assays. Patients (N=1,298) were grouped based on TAF or TDF treatment and stratified as oral antiviral (OAV) naïve or experienced (TAF: OAV-naïve, n=669; OAV-experienced, n=197; TDF: OAV-naïve, n=333; OAV-experienced, n=99). Most enrolled patients were infected with WT HBV at baseline, mutations were 2–3 times more common in OAV-experienced patients compared with OAV-naïve, and primary mutations were detected in 1–2% of OAV-naïve and 20–25% of OAV-experienced patients. In total, 38 patients qualified for sequence analysis through Week 48, of which 45% (n=17) were non-adherent to the study drug. Sequence changes observed through Week 48 are shown in Table 2. In the TDF group, two patients had conserved site changes (rtQ67Q/H and rtQ288Q/stop) although most patients had no sequence changes compared with baseline or were unable to be sequenced. Phenotypic analyses of patients who experienced virological breakthrough (VB) (TAF, n=5; TDF, n=4) and those in the TDF group that had conserved site mutations (n=2), showed that all isolates remained sensitive to TFV. Deep-sequence analysis was carried out on 16 patients, and 30 different emerged or enriched substitutions were detected; 28 of these were observed in one patient only, while two polymorphic substitutions (rtH123D and rtN124D) each occurred in two patients. Polymorphic substitutions, rtH123D and rtN124D, were observed in two patients, each of the four patients with these mutations, and three had VB-associated with non-adherence (all three remained on the study drug and achieved HBV DNA <69 IU/mL). One adefovir dipivoxil-resistance-associated substitution was detected in a patient in the TDF group.
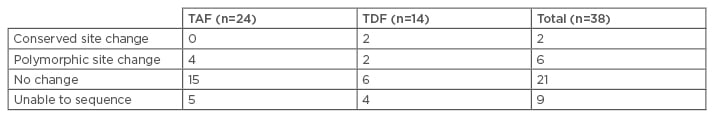
Table 2: Sequence changes observed through Week 48.
TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate.
Similar numbers of patients in the TAF and TDF groups qualified for resistance analysis through Week 48, although no resistance to TAF was detected. VB was mostly associated with non-adherence to the study drug and no substitutions detected by population or deep sequencing were associated with sustained VB.
Safety and Efficacy of GS-9620 in Virally-Suppressed Patients with Chronic Hepatitis B
Toll-like receptor 7 (TLR7) is a pattern-recognition receptor located within plasmacytoid dendritic cells (pDCs) and B cells, of which viral single-stranded RNA is the natural ligand;21 TLR7 activation results in innate and adaptive immune stimulation through secretion of IFN by pDCs, increased expression of molecules associated with antigen presentation and T cell co-stimulation, and B cell differentiation to immunoglobulin-producing plasma cells.22-24 GS-9620 is a TLR7 agonist that is designed for pre-systemic activation of TLR7 to minimise systemic exposure and side effects from the drug or induced cytokines.
Dr Harry Janssen,25 University Health Network, Toronto, Canada, presented the results of a Phase II safety and efficacy study of GS-9620 in patients with CHB infection suppressed on OAV treatment. Patients were divided into three cohorts: cohort A: n=52, GS-9620 for 4 weeks; cohort B: n=57, GS-9620 for 8 weeks; cohort C: n=53, GS-9620 for 12 weeks, and stratified by HBeAg status and HBsAg level, and randomised 1:3:3:3 to placebo, GS-9620 1, 2, or 4 mg, respectively. Baseline demographics were comparable between placebo and treatment groups. Adverse events (AEs) did not show dose dependency across cohorts and a similar proportion of patients reported any AEs in each cohort. Two serious AEs were experienced in the 2 mg and 4 mg cohorts each, compared with none in the 1 mg and placebo cohorts. Likewise, Grade 3 and 4 laboratory abnormalities were comparable across cohorts. Dose-dependent induction of interferon-stimulated gene 15 (ISG15) was observed and male gender was associated with a lower probability of ISG15 induction, whereas HBeAg+ and higher baseline CD20% trended to association with ISG15 induction, p=0.059 and p=0.067, respectively. It is worth noting that removing the single outlier resulted in a significant relationship. HBsAg changes were minimal across all cohorts; no patients had >0.5 log10 declines or HBsAg loss at Week 24. Two patients had HBeAg loss at Week 24; both of whom were in cohort B. There was no association of 24-hour ISG15 change with HBsAg-level change reported at Week 24.
Overall, GS-9620 was safe and well-tolerated in patients with CHB, and consistent dose-dependent pharmacodynamic induction of ISGs was demonstrated, although HBsAg declines were not significant. Further investigation in treatment-naïve patients with CHB is underway.
Features of the Metabolic Syndrome are Associated with Lack of Serum ALT Normalization During Therapy for Chronic Hepatitis B
TAF is a new TFV prodrug that enhances delivery of active drug to hepatocytes, however there are limited data on factors that affect ALT normalisation. Lack of ALT normalisation may be associated with obesity-related liver disease in patients with CHB infection.26
Dr Scott Fung,27 University Health Network, presented the findings of two Phase III trials exploring the factors associated with ALT normalisation in patients with CHB treated with TAF or TDF (including those with compensated cirrhosis). Patients were randomised 2:1 and stratified by HBV DNA level and treatment status (naïve versus experienced). Baseline demographics, disease characteristics, and medical history were comparable between the two treatment groups. Rates of ALT normalisation over 72 weeks were higher with TAF versus TDF with both AASLD and Central Laboratory criteria. Subsequent analysis was conducted using primary endpoint data at Week 48. Analysis revealed that patients with ALT levels greater than the ULN by AASLD criteria had features of metabolic syndrome (high BMI, hyperlipidaemia, and hypertension). Patients without risk factors for metabolic syndrome treated with TAF had higher rates of ALT normalisation than those treated with TDF (TAF, n/N=248/439, 57% versus TDF, n/N=89/212, 42%; p<0.001) and ALT normalisation rates decreased with increasing number of risk factors. Factors associated with a lack of ALT normalisation by AASLD criteria at Week 48 included baseline BMI and ALT, HBV DNA <29 IU/mL, female gender, cirrhosis, TAF 25 mg, and diabetes.
TAF treatment was associated with higher rates of ALT normalisation compared with TDF treatment at Weeks 48 and 72, and patients with metabolic syndrome were less likely to achieve normalisation following TAF or TDF treatment.
Correction of Early Biochemical and Virologic Responses During Oral Antiviral Therapy for Chronic Hepatitis B
In patients with CHB, the 12-week post-treatment time point is important for antiviral therapy and is predictive of non-response to nucleoside analogue therapy, according to current practice guidelines.28
Dr Maurizia Brunetto,29 Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, presented the findings of two Phase III trials evaluating rapid biochemical and virological responses in patients with CHB infection treated with TAF (N=866) and TDF (N=432). Enrolment included patients with compensated cirrhosis and patients were randomised 2:1 and stratified by HBV DNA level and treatment status (naïve versus experienced). At 12 weeks, 24% of TAF patients had achieved HBV DNA suppression, compared with 22% of TDF patients. ALT normalisation, according to AASLD criteria, was achieved in 18% and 11% of TAF and TDF patients, respectively (p=0.003). In the TAF group, 9% (75/831) of patients achieved both ALT normalisation and virological response at Week 12, compared with 5% (21/416) in the TDF group. HBV DNA suppression was associated with ALT normalisation; more patients achieved ALT normalisation with TAF (40%, 75/190) compared with TDF (24%, 21/89), p=0.01. HBV DNA suppression and ALT normalisation at Week 12 were associated with lower baseline HBV DNA, HBeAg negativity, and male gender, and TAF treatment was independently associated with achieving this endpoint.
A minority of patients achieved Week 12 ALT normalisation and virological suppression, of which a greater proportion were in the TAF group versus TDF group. Treatment with TAF, in addition to host and viral baseline features, was associated with Week 12 ALT normalisation and HBV DNA suppression.
Genotype Specific Differences in Magnitude of HBsAg Reduction During TDF or Tenofovir Alafenamide Therapy in CHB Patients
HBsAg loss or seroconversion is the treatment goal for CHB therapies, but is rarely achieved with current therapy.30 Current therapies achieve viral suppression, which has been shown to reduce long-term complications, however HBsAg seroclearance is a more important biomarker for prevention of HCC and is associated with decreased long-term morbidity and mortality.31
Dr Patrick Marcellin,32 Hôpital Beaujon, University of Paris, Clichy, France, presented the findings of two Phase III studies that evaluated the 48-week HBsAg kinetics of TAF (N=866) versus TDF (N=432) in treatment-naïve and treatment-experienced patients with CHB. In the pooled analysis, mean change from baseline to Week 48 in HBsAg was comparable between TAF and TDF (-0.31 versus -0.34 log10 IU/mL, respectively). Mean change from baseline to Week 48 in HBsAg was -0.078 log10 IU/mL and -0.442 log10 IU/mL for HBsAg– and HBsAg+ patients, respectively. HBeAg+ patients had greater HBsAg declines that increased with duration of treatment, whereas HBeAg– patients showed minimal decline in HBsAg. Patients with HBV genotypes A and B had similar HbsAg >0.5 log10 reduction at Weeks 24 and 48, and the lowest rates were observed in HBV genotypes C and D, both regardless of HBeAg status. HBeAg+ HBV genotype A and B patients showed significantly greater declines in HBV DNA compared with genotype C and D patients (-6.3 and -6.1 versus -5.7 and -5.7, respectively, p<0.005).
Overall, patterns of HBsAg decline by genotype were similar across HBeAg+ and HBeAg– patients and changes were similar for TAF and TDF treatment groups across genotypes. HBV genotypes B and D were associated with a higher and lower probability of achieving >0.5 log10 reductions in HBsAg, respectively.
CONCLUSION
The Viral Hepatitis and Orthotopic Liver Transplant poster session, focussing on HBV, consisted of a range of studies relating to different aspects of HBV and CHB infection. The epidemiology and natural history of HBV was studied prospectively in adults presenting for outpatient endoscopy and it was concluded that the majority of patients were at a high risk of CHB and that targeted education is a necessity. Several posters focussed on the immunology and virology of HBV, in particular on viral variation and resistance, immune recovery and immune markers, and viral antigens. The following conclusions have been drawn and collated from data presented in a number of posters from this session. Overall, no resistance was detected in patients treated with TAF, the role of early expression of HBx was elucidated, HBsAg and HBcAg expression was varied across patients, and the progression of disease from IC to IR was associated with variations in the BCP and PC regions of HBV. Treatment of HBV with TFV (TAF and TDF) included Phase II and III studies on safety and efficacy, ALT normalisation, and virological responses to treatment. It was found that GS-9620 was well-tolerated in patients with CHB, that TAF was associated with ALT normalisation and HBV DNA suppression, with the exception of patients with metabolic syndrome (who were less likely to achieve normalisation), and HBsAg declines were consistent across HBV genotypes and HBeAg status.
CHRONIC HEPATITIS B APPROVED THERAPIES ORAL SESSION
With the introduction of the HBV vaccine and other preventive measures, the global prevalence of infection has significantly decreased, however CHB infection remains a challenging global health problem. More than 350 million chronically infected individuals are at risk of hepatic decompensation, cirrhosis, HCC, and death.33 While a variety of treatment options are available to manage patients at different disease stages, no definitive cure exists. Thus, clinicians and researchers are tasked with identifying potential new treatments and uncovering knowledge that improves outcomes. At the AASLD 2016 Liver Meeting, several related presentations were given by top researchers, of which we summarise three. The first presentation, by Dr Wai-Kay Seto, Department of Medicine, University of Hong Kong, Hong Kong, investigated changes in bone outcomes in two formulations of the commonly used TFV for treatment of CHB.34 In the second presentation, Prof George Papatheodoridis, Medical School of Athens University, Athens, Greece, reported results on potential survival factors in Caucasian patients with CHB,35 and lastly, Dr Peng Hu, Department of Infectious Diseases, The Second Affiliated Hospital of Chongqing Medical University, Chongqing, China, revealed the final analysis of the NEW SWITCH trial, which evaluated the effect of a non-conventional treatment regimen on HBsAg loss and seroconversion.36
Reduced Changes in Bone Mineral Density in Chronic HBV Patients Receiving Tenofovir Alafenamide (TAF) Compared with Tenofovir Disoproxil Fumarate (TDF)
TDF is an established nucleotide analogue that is used as first-line treatment in the management of CHB.28,37 While generally considered safe and well-tolerated,33,38 the potential toxicities associated with long-term TDF therapy may cause concern and pose a major drawback to prolonged use.39 Accordingly, investigators have been exploring alternative formulations that provide efficacy along with an improved safety profile relative to the standard TFV treatment approach. The recent approval of a TAF formulation has provided clinicians with one such alternative treatment that has an efficacy and a safety profile that is improved over TDF standard of care.40
Of particular importance to chronic TDF therapy are the potential adverse effects on bone mineral density (BMD). Randomised clinical trials and observational case studies show that TDF treatment is associated with bone loss, osteopenia, and increased fracture risk.41-44 The aim of the study presented by Dr Seto was to explore factors associated with changes in BMD over 48 weeks in patients with CHB treated with TAF compared with TDF. To this end, Dr Seto explained that the study comprised a cohort of patients from two recently published randomised, double-blind, controlled trials that investigated the efficacy and safety of TAF versus TDF in HBeAg+ (NCT01940471)45 and HBeAg– (NCT01940341)46 patients. Patients were randomised in a 2:1 fashion to either TAF or TDF for a duration of up to 144 weeks. Bone outcomes were measured using dual energy X-ray absorptiometry (DXA) scans at baseline and every 24 weeks up to 144 weeks, then annually. FRAX® scores were also obtained at baseline and at various times throughout the study; FRAX scores use a computer-based algorithm that integrates several well validated fracture risk factors to obtain the 10-year probability of hip fracture and major osteoporotic fracture.47 Other bone endpoints measured included the percent change in hip and spine BMD at Weeks 48 and 72, shifts from baseline in T-scores at Week 48, and change in FRAX score and category at Week 48.
Of the patients randomised to either TAF or TDF, baseline demographics and disease characteristics were comparable. When bone outcomes were analysed using DXA, 48 and 72 weeks of TAF treatment resulted in better outcomes compared with TDF. TAF-treated patients had significantly smaller declines in hip and spine BMD, and fewer patients’ baseline BMD shifted from normal to osteopenic, or osteopenic to osteoporotic. To determine whether the improved bone outcomes with TAF depended on fracture risk, patients were stratified by the number of risk factors (e.g. female gender, age ≥50 years, Asian race, impaired renal function) and by baseline FRAX score. Regardless of the number of risk factors, fewer TAF-treated patients had significant (>3% BMD decrease) reductions in BMD compared with TDF treatment. In fact, the percentage of patients in the TAF- treated group who showed a significant decrease stayed around 10%, whereas TDF-treated patients increased from approximately 20% to 60% with an increase from 1 to 4 risk factors, respectively. Similarly, the risk of hip BMD decline for patients on TAF remained almost unchanged and just below 10% with increasing FRAX score. Conversely, the percentage of TAF-treated patients with a significant decrease in BMD grew with increasing baseline FRAX score. These findings suggest that TAF imparts significantly fewer adverse effects on bone parameters compared with TDF in patients with CHB up to 48 weeks.
Hepatocellular Carcinoma (HCC) is the Only Factor Affecting the Excellent Survival of Caucasian Chronic Hepatitis B (CHB) Patients with or Without Cirrhosis Under Long-Term Entecavir (ETV) or Tenofovir (TDF) Therapy
ETV and TDF are standard of care, first-line treatments for CHB that maintain long-term viral suppression in >95% of patients, improve liver histology, and often reverse histological cirrhosis.28,38,48-51 While these treatments have improved outcomes for patients with CHB, their effect on survival remains unclear. Long-term complications of infection include cirrhosis and HCC, which together are responsible for >700,000 deaths annually.52,53 Among patients with active viral replication, cirrhosis will develop in 15–20% within 5 years; and in cirrhotic patients, the 5-year probability of decompensation is 15–20%.54 After patients progress to decompensated cirrhosis, the prognosis is poor; the 5-year survival rate is only 14%.54 The aim of the ongoing, large-scale, 10-centre study summarised here was to evaluate the probability of survival and factors affecting survival in Caucasian patients with CHB who have been treated with long-term ETV/TDF therapy.
The study included nearly 2,000 patients either NUC naïve or experienced, aged ≥16 years, with or without compensated cirrhosis, and treated with ETV or TDF for ≥12 months; approximately 80% of patients in the study received ETV or TDF monotherapy. Patients were excluded if they had a HCC diagnosis prior to treatment, decompensated cirrhosis, hepatitis D virus/hepatitis C virus/HIV coinfection; or prior liver transplant (LT). Follow-up was considered as the time between the onset of ETV/TDF therapy and the date of death or LT, or the last available clinical information, and included clinical, biochemical, and virological evaluations every 6 months.
When the absolute 10-year outcomes were evaluated, a significantly higher percentage of cirrhotic patients compared with non-cirrhotic patients was observed for the following outcomes: death from any cause, liver-related and unrelated deaths, LT, and HCC. Overall survival (OS) among all patients stabilised just below 95% at 7 years following initiation of ETV/TDF therapy; however, a statistically significant (p<0.001) difference in OS emerged when patients with CHB with and without cirrhosis were compared. The cumulative probability of OS in cirrhotic versus non-cirrhotic patients at 5 years was approximately 93% versus 97%, respectively, and 89% versus 96% at 10 years, respectively. When patient characteristics were evaluated in Cox regression analyses, univariate analysis showed that increasing age, cirrhosis, and the presence of HCC were associated with an increased risk of death, and that platelet count and past peg-IFN-α treatment were associated with a decreased risk of death. In the multivariate analysis, only cirrhosis was not significant and all other variables remained independent predictors of mortality. The cumulative probability of survival from liver-related death, transplant-free survival from liver-related death, survival in patients without HCC, and transplant-free survival from liver-related death in patients without HCC was also significantly higher (p<0.001) in patients without cirrhosis. Conversely, cirrhosis did not predict differences in survival among patients with HCC.
Together, the results demonstrate that in Caucasian patients with CHB with or without compensated cirrhosis and treated with long-term ETV/TDF therapy, OS is excellent (>95% at 5 years); a significant proportion of deaths come from liver unrelated causes, and HCC is the major factor affecting the OS and the only factor affecting transplantation-free liver-related survival.
Increased and Sustained HBsAg Loss in HBeAg Positive CHB Patients Switched From NUC to Peg-IFN Alfa-2a: A Randomised Open Label Trial (NEW SWITCH Study)
Antiviral therapy using NUC therapy is the standard of care for patients with CHB.28,48 While antiviral drugs can effectively suppress HBV replication long-term during treatment and improve some histological outcomes, they usually do not lead to HBsAg loss, which is the ideal endpoint as it suggests virological cure.38,49-51 Thus, various treatment approaches are being evaluated with the goal of promoting HBsAg loss. Strategies using various sequences and combinations of NUC with peg-IFN-α are currently under investigation. NUC in combination with peg-IFN-α, adding IFN to suppressive NUC therapy, or switching from NUC to TDF may increase the likelihood of HBsAg loss, but the optimal treatment strategy is not yet clear.55-57 The NEW SWITCH study aims to provide insight on one such strategy.
In the multicentre, open-label NEW SWITCH study, 305 HBeAg+ Chinese patients with CHB treated with lamivudine, ETV, or adefovir for 1–3 years with partial response (HBV DNA <200 IU/mL and HBeAg loss) were randomised to treatment with combination NUC and peg-IFN-α therapy for 12 weeks followed by an additional 36 or 84 weeks of peg-IFN-α monotherapy (randomisation 1:1 to a total 48 or 96 weeks of treatment, respectively). HBsAg loss and seroconversion were compared at 48 weeks, at the end of treatment (EOT) and at end of follow-up, which occurred 48 weeks after the EOT.
An interim Week 48 analysis of the overall population showed that 13.2% had achieved HBsAg loss and 12.2% experienced HBsAg seroconversion. However, dramatic differences in HBsAg loss were observed in patients when stratified according to baseline HBsAg levels. Patients with lower HBsAg at baseline (<1,500 IU/mL) were significantly more likely to achieve HBsAg loss than those with higher baseline levels (25.4% versus 3.0%; p<0.0001). Furthermore, patients whose HBsAg decreased to <200 IU/mL by Week 24 after switching to peg-IFN-α monotherapy also had a higher rate of HBsAg loss at Week 48, compared with patients with HBsAg ≥200 IU/mL at Week 24 (40.4% versus 0.0%, respectively; p<0.0001). Extending the duration of peg-IFN-α from 48 to 96 weeks resulted in a higher percentage of patients who showed HBsAg loss (20.7% versus 14.4%) and HBsAg seroconversion (16.0% versus 13.1%) at 96 weeks; albeit not reaching statistical significance (p>0.05). The benefits of longer peg-IFN-α treatment on HBsAg loss (15.3% versus 9.8%) and seroconversion (12.0% versus 9.2%) appeared to extend through to the end of follow-up (Week 144). Of the patients who showed HBsAg loss at the EOT, about 64% showed sustained HBsAg loss at the end of follow-up. However, the presence of HBV DNA in some patients who previously demonstrated HBsAg loss suggested reactivation. In addition to efficacy, safety was also measured in the two treatment arms: 3 patients experienced an ALT flare (defined as ALT >5x ULN) at the EOT and 2 at the end of follow-up; 43 (14.2%) patients experienced virological relapse (defined as HBV DNA 2,000 IU/mL). Increased treatment duration did not significantly increase the risk of adverse effects.
These results suggest that using peg-IFN-α with or after NUC analogues increases HBsAg loss rate. Prolonged treatment duration of peg-IFN-α may be associated with greater likelihood of achieving HBsAg loss and seroconversion. Additionally, patients who start with lower HBsAg levels have a better chance of sustained HBsAg clearance, which may aid in selecting patients who are more likely to be cured with the addition of IFN.








