Meeting Summary
Prof Nezam Afdhal provided a background to thrombocytopenia (TCP) in chronic liver disease (CLD). He explored the causes of TCP and discussed what are considered acceptable platelet levels. He described the delicate balance between thrombosis risk and bleeding risk that puts CLD patients with TCP at risk of complications, particularly when they require invasive procedures.
Through a series of case studies, the faculty highlighted current management dilemmas and novel approaches to TCP management. Prof Edoardo Giannini presented the case of a patient with hepatocellular carcinoma (HCC) (platelet count of <50×109/L) who was given a platelet transfusion prior to radiofrequency thermal ablation (RFTA). The patient’s increase in platelet count was not clinically significant; therefore, the procedure was cancelled. Prof Giannini noted that radiology guidelines state that for procedures with a moderate risk of bleeding (such as RFTA), platelet transfusion is recommended for counts <50×109/L.
Prof Mark Thursz presented a case of a nonalcoholic steatohepatitis and refractory ascites, in which the patient had a number of large-volume paracentesis procedures. He then presented paracentesis studies highlighting that bleeding events are often unrelated to patients’ platelet levels. Prof Giannini described a study in patients with acute-on-chronic liver failure (AoCLF) who underwent paracentesis and in whom the bleeding rate was 3%.
Following these case presentations, Prof Markus Peck-Radosavljevic discussed the role of thrombopoietin (TPO) in TCP in CLD. He then examined the pivotal trials of various TPO-receptor (TPO-R) agonists which have been studied in CLD patients with TCP undergoing invasive procedures. Clinical studies of the TPO-R agonist lusutrombopag included a large proportion of high-risk bleeding patients and therapy with this agent has been shown to elevate platelet count levels for up to 2 weeks, allowing a window in which to schedule invasive procedures.
Introduction
Professor Nezam Afdhal
Prof Afdhal opened the symposium, which took the format of a debate on the management of TCP in CLD.
TCP in CLD is common. Many factors can lead to the development of TCP in CLD, but there are two primary modalities: portal hypertension with associated hypersplenism, leading to both the sequestration and destruction of platelets, and the decreased levels or activity of TPO. Depending on the aetiology of the liver disease, other co-factors may also be at play; for example, HCC and chemotherapy, autoimmune disease (common in hepatitis C [HCV]), and antiviral therapy can also induce TCP.1,2
Decreased platelet production is usually due to low TPO levels, which results in reduced bone marrow production of platelets. Various clinical factors can impact this; alcohol, for example, is a well-known suppressant of platelet production.3
Chronic TCP is usually related to a slow decline in platelet production (i.e., the sequestration and destruction that occurs with progressive fibrotic cirrhotic liver disease). Platelet levels decrease as the liver progresses to cirrhosis. Similar changes over time can be seen in TPO levels.4,5
Coagulation is one of the most important functions of the liver. Many of the proteins and co-factors necessary for adequate haemostasis may be decreased in cirrhosis, including decreased production of procoagulants, coagulation factors, fibrinogen, and platelets, as well as increased levels of von Willebrand factor. Some diseases are associated with alterations in anticoagulants, such as proteins C and S and antithrombin 3, resulting in an increased risk of thrombosis.1,6,7
Consequently, in liver disease the balance between risk of bleeding and risk of thrombosis is disturbed.6,8
Impact of Thrombocytopenia on Bleeding Risk
The HALT-C trial in HCV was a 5-year study that examined the ability of low-dose interferon to prevent the progression of cirrhosis. Multiple repeated liver biopsies were performed on patients and bleeding risk was 0.6%. This percentage increased to 5.3% in patients with a platelet count of ≤60×109/L,9 reaching levels that begin to define TCP.
A platelet count of ˜60×109/L platelets will maintain thrombin generation at the 90th percentile of normal in patients with cirrhosis. Below ˜60×109/L, thrombin generation is impaired (Figure 1).10
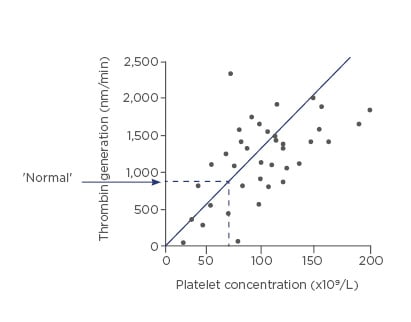
Figure 1: Platelet count of ˜60×109/L maintains thrombin generation at the 90th percentile of normal.
Adapted from Tripodi et al., 200610
In conclusion, the balance between bleeding and clotting is extremely important in treating patients with cirrhosis. The causes can be multifactorial and must be evaluated by the clinician.
Debating the Management of a Chronic Liver Disease Patient with Thrombocytopenia Undergoing a Procedure: Case 1
Professor Edoardo Giannini
Prof Giannini presented the case of a 69-year-old male with a history of diabetes and arterial hypertension. In 2002, he was diagnosed with HCV and, following a liver biopsy, he was diagnosed with advanced fibrosis. Treatment with pegylated interferon was ineffective. In 2015, he presented with ascites and was treated with spironolactone. Endoscopy revealed small oesophageal varices. The patient was Child–Pugh Class B. He was treated with sofosbuvir/daclatasvir/ribavirin for 24 weeks and had a sustained virological response.
During follow-up, diuretics were withdrawn. In 2018, a 2.1 cm liver focal lesion (S5) was identified. A MRI scan diagnosed HCC. The patient’s liver function was preserved but he had slightly altered renal function, mainly due to hypertension and diabetes. His alpha-fetoprotein was slightly altered (14 ng/mL) and his international normalised ratio (INR) was slightly prolonged (1.35). His platelet count did not improve after antiviral therapy (41×109/L). His model for end-stage liver disease (MELD) score was 14 and he was Child–Pugh Class A. The multidisciplinary team discussed his case and decided to proceed with RFTA.
Prof Giannini referred to a study in which 4,133 RFTA procedures were performed in patients (mainly diagnosed with HCC) with a platelet count >50×109/L. In this study, 1.5% of patients experienced bleeding and TCP was deemed to be a bleeding risk factor.11 Another study in HCC patients where 1,843 RFTA HCC procedures took place highlighted that 10 platelet packs were transfused in patients with a platelet count <50×109/L. The proportion of patients who bled in this study was 0.5%.12
Prof Giannini discussed the prevailing expert opinion in pre-procedure prophylaxis. Opinions include that platelet counts “below <50×109/L may be associated with a higher risk of bleeding” and “thrombopoietin agonists may have a role in pre-planned procedural prophylaxis.”13 In a Spanish survey, 88.8% of healthcare professionals stated they would correct haemostatic abnormalities, based on platelet counts, if there was a moderate (3–10%) risk of bleeding. In total, 77.3% of responders thought that 26–50×109/L was the appropriate platelet count range in which to take action to decrease the risk of bleeding.14 He noted that, in an Italian study, platelet count did not increase in a clinically significant manner following platelet transfusion.15
Returning to the clinical case, Prof Giannini said that there was a modest increase in platelet count following platelet transfusion, but levels did not increase sufficiently to allow RFTA.
Prof Giannini summarised by stating that RFTA in HCC carries a moderate risk of bleeding. Severe TCP may be associated with an increased bleeding risk and can result in delayed or cancelled procedures. Prophylactic platelet transfusions are commonly used, although they are controversial, and a threshold of >50×109/L pre-procedure is generally accepted.
Case 1 (Rebuttal)
Professor Mark Thursz
Prof Thursz began his case rebuttal by reviewing current guidelines on platelet levels and prophylactic approaches to managing bleeding risk. He noted that no guidelines exist for RFTA. For liver biopsy, the American Association for the Study of Liver Diseases (AASLD) states that platelet transfusion should be considered when levels are <50–60×109/L, the British Society of Haematology (BSH) says the range is <50–60×109/L, and the British Society of Gastroenterology (BSG) states that biopsy can be performed safely if platelet levels are >60,000/mm3.16,17
A case series was presented in which, counterintuitively, the rates of post-procedural bleeding were higher in patients with platelet counts of >50×109/L compared to patients with ≤50×109/L.18
Prof Thursz noted that platelet level may not be the only parameter to consider, as platelet function may also be impaired in liver cirrhosis.19 Clinicians have to weigh up procedural risk (by analysing bleeding time), consider platelet level and function, and assess potential response to platelet transfusion versus the risk.
Case 1 (Response)
Professor Edoardo Giannini
Prof Giannini pointed out that in the case series Prof Thursz presented, the 13% of patients with severe TCP were diluted into the series and this series also included patients who had a very low risk of bleeding. While he agreed that platelet function is an important consideration, he stated that it is not possible to assess it in a meaningful way.
The Society of Interventional Radiology (SIR) guidelines state that, in procedures with a moderate risk of bleeding (such as RFTA), platelet transfusion is recommended for counts <50×109/L. Prof Giannini noted that the risks and limitations of platelet transfusions include refractoriness, high cost, limited availability, risk of transmission of infection, limited efficacy, and transfusion-associated lung injury.
Debating the Management of a Chronic Liver Disease Patient with Thrombocytopenia Undergoing a Procedure: Case 2
Professor Mark Thursz
Prof Thursz presented the case of a 74-year-old male who presented in 2016 with abdominal swelling, a past history of poorly controlled diabetes, and a metallic aortic valve replacement. The patient had hypertension and hyperlipidaemia and was taking warfarin, metformin, candesartan, and atorvastatin.
The patient had features of CLD and features of liver failure (gross ascites and ankle oedema). He was anaemic, his platelet levels were 121×109/L, and his INR was raised, possibly due to warfarin.
The patient was screened for hepatitis. His ultrasound (US) and CT scans both showed gross ascites, an irregular liver edge, cirrhosis, abdominal varices, an enlarged portal vein, an enlarged spleen, and no focal lesions.
He had decompensated cirrhosis (MELD score of 18; Child–Pugh score of 8 [Class B]) and probable nonalcoholic steatohepatitis as the underlying diagnosis. The patient underwent a successful large volume paracentesis and was discharged on diuretic therapy.
A few months later he was re-admitted with encephalopathy, hyponatraemia, and diuretic-resistant ascites. His haemoglobin and platelet levels (79×109/L) had dropped. Renal function had deteriorated slightly. There was no evidence of bacterial peritonitis.
The patient met the European Association for the Study of the Liver (EASL) diagnostic criteria for refractory ascites.20 The patient underwent a large volume paracentesis, warfarin was reversed, and he received fresh frozen plasma (FFP). The patient deteriorated quickly after the procedure, requiring surgical repair of lacerated-wall varices, and diuretics were discontinued. The patient was discharged and another large volume paracentesis was planned. Three months later, he was re-admitted with tense ascites, an INR of 2.5, and a platelet count of 35×109/L. Six weeks later, the patient developed fatal portal vein thrombosis.
Prof Thursz presented data from a number of studies in CLD patients requiring paracentesis, one of which examined the haemorrhage risk of US-guided paracentesis (3,116 procedures).21 Haemorrhage occurred in 6 (0.19%) procedures and was not related to INR or platelet count. Using this study as an example, Prof Thursz noted that not correcting coagulopathy could have saved 1,125 units of FFP and 366 units of platelets at a cost of $816,000. He noted that thromboelastography-guided transfusion made no difference to the risk of bleeding in patients receiving invasive procedures.22 In 4,729 patients who underwent paracentesis, 9 (0.19%) had a haemorrhage after the procedure. Most of these patients had reasonable platelet levels and INR.23
Prof Thursz therefore concluded that paracentesis is a low-risk procedure in which the main cause of bleeding is typically procedural trauma.
Case 2 (Rebuttal)
Professor Edoardo Giannini
Prof Giannini felt that Case 2 was an example of a patient in which a pendulum was swinging between thrombosis and bleeding (Figure 2).
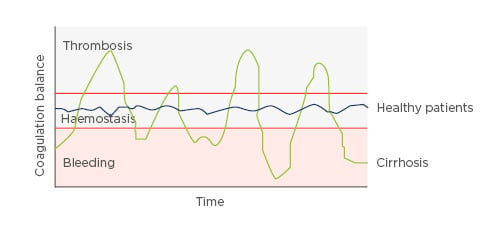
Figure 2: The ‘ups and downs’ of coagulation in a patient with cirrhosis.
Factors such as infection, alcohol use, or other external factors that had not been identified could tip the balance either way.
He described a study that analysed the bleeding rate and risk factors following paracentesis in patients with AoCLF; bleeding rate was 3%, over 10-times higher than that described in the study Prof Thursz presented. In this study, the haemostatic parameters seemed to play a role.25
Prof Giannini also described a recent study in which 60% of AoCLF patients at admission had a hypocoaguable profile compared to 30% of acute decompensated patients. In a secondary analysis of data, patients with a hypocoaguable profile were more frequently bleeding at admission, and more often had to receive transfusion of red blood cells, FFP, and platelets; these are factors that appear to be associated with a decreased survival of patients and an increase in mortality.26 Prof Giannini added that unstable patients may tend towards a hypocoaguable state, meaning their bleeding tendency may be increased.
Case 2 (Response)
Professor Mark Thursz
Prof Thursz responded that the data set he cited related to several thousand patients, while Prof Giannini only referred to a study in several hundred patients. He restated that in paracentesis, the bleeding risk is very low and is not associated with the platelet count, but with the procedure. He noted that platelet transfusions are difficult to arrange when patients present as emergencies. He also believed that the safety concerns of platelet transfusions are underestimated.
Emerging Thrombopoietin-Receptor Agonists for the Management of Thrombocytopenia in Chronic Liver Disease Patients Undergoing a Procedure
Professor Markus Peck-Radosavljevic
TPO is the predominant endogenous thrombopoietic growth factor and is produced in the liver. While several cytokines are involved in thrombopoiesis, TPO plays a role across the platelet production pathway and is the most crucial and specific growth factor for platelet production. Reduced TPO production is a major factor in TCP in CLD and cirrhosis patients.27
Small molecule TPO-R agonists are capable of binding to the TPO receptors which activate the downstream signalling cascade to stimulate platelet production.28 The first TPO-R agonist to be studied in liver disease was eltrombopag. In a study of 292 patients with cirrhosis with platelet counts of <50×109/L, treatment with eltrombopag increased platelet count; however, due to an excess of portal vein thrombosis in the treatment group versus the control group, the study was terminated early, and this drug is not used in the management of TCP in CLD.29
Another TPO-R agonist, avatrombopag has been evaluated in two Phase III trials (ADAPT-1 and ADAPT-2)30 in patients with cirrhosis undergoing invasive procedures. The studies assessed two different doses: one for very severe TCP patients (platelet levels <40×109/L) and one for patients with 40–50×109 thrombocytes/L. Treatment was given for 5 days and the procedure was performed at Day 10. Both studies included a high number of patients undergoing low-risk bleeding procedures, such as endoscopy (52%). Some 12.7% of patients underwent moderate-risk procedures (e.g., chemoembolisation for HCC), and 9.6% and 7.8% underwent high-risk dental procedures and RFTA, respectively. Both trials had positive outcomes.
There have also been two Phase III studies of lusutrombopag: L-PLUS 1 and 2. Both trials had similar designs and patients’ baseline platelet count was <50×109/L. Treatment was given for up to 7 days, depending on platelet count level at Day 5. The trials included additional safety checks: the portal vein was analysed via US or CT scan before and after the drug was given. No other TPO-R agonist drug trials have this much detailed information about non-clinically apparent portal vein thrombosis. Platelet transfusions were administered in patients who did not reach a platelet count of <50×109/L.31,32
In L-PLUS 1, a high proportion of patients underwent procedures that had a significant risk of bleeding, such as RFTA/microwave coagulation therapy and transarterial chemoembolisation. The L-PLUS 2 study had a high proportion of procedures, such as endoscopy and dental extraction. Overall, the risk of bleeding in the lusutrombopag trials was higher than in other trials. In L-PLUS 1, the primary endpoint (proportion of patients not requiring platelet transfusion prior to invasive procedure) was achieved by 79.2% of lusutrombopag patients compared to 12.5% of placebo patients. In L-PLUS 2, the primary endpoint (proportion of patients not requiring transfusion prior to invasive procedure, and no rescue therapy for bleeding, from randomisation through 7 days after invasive procedure) was achieved by 64.8% of lusutrombopag patients compared to 29% of placebo patients (Figure 3).31,32
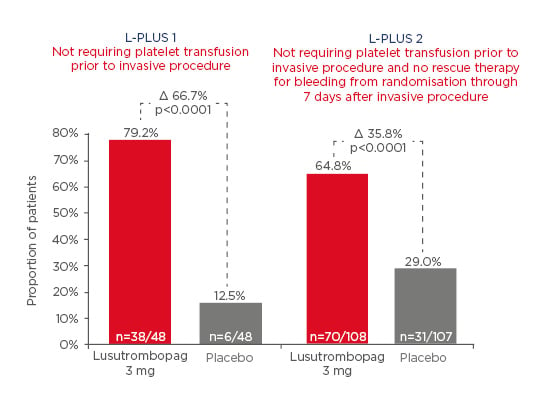
Figure 3: Primary endpoints in the L-PLUS 1 and L-PLUS 2 trials.
Lusutrombopag therapy resulted in up to 2 weeks of elevated platelet counts, providing an opportunity for repeat procedures. There were almost twice as many bleeding events in the placebo group (27.1%) compared to the lusutrombopag group (14.6%). In both studies, there was no difference in thrombotic events between lusutrombopag (3 events) and placebo (3 events).31,32
Discussion
During the discussion, the faculty answered questions from the audience, considering clinical circumstances that can affect a patient’s coagulation status and bleeding risk. For example, Prof Giannini noted that renal dysfunction and infection in AoCLF may tip the balance in favour of a hypocoagulable state and make patients more prone to bleeding. Prof Afdhal explained that elevated creatinine can be associated with a higher risk of bleeding in patients undergoing paracentesis.
Prof Peck-Radosavljevic described how he would manage a patient with Budd–Chiari syndrome who has underlying thrombophilia. He would begin with anticoagulation therapy to improve portal hypertension. He said paracentesis would then often not be required. A transjugular intrahepatic portosystemic shunt could then be inserted; this will usually remove the issue of repeat paracentesis. He would not give a platelet transfusion or an agent that stimulates platelet production in a case with a distinct procoagulatory state.
In terms of the impact of drug therapies in bleeding risks, Prof Thursz suggested that newer anticoagulant drugs can have bleeding risks similar to that of warfarin. He said that monitoring thrombin generation in patients with very advanced liver disease may not be helpful.
Prof Afdhal highlighted the importance of the duration of the effect achieved by platelet transfusion therapy. Cirrhotic patients who undergo polypectomy have a high risk of secondary bleeding. Prof Peck-Radosavljevic explained that secondary bleeding can usually happen up to Day 7, which means this time period is covered with a TPO-R agonist but would never be covered by a platelet transfusion.
Prof Thursz thought that in pre-planned procedures, TPO-R agonists should be used in RFTA, in procedures done by endoscopists, such as a polypectomy, and dental procedures. He noted that dental sepsis can be an issue for patients awaiting liver transplant. Dental extractions have a high risk of bleeding because of the infection around the root of the tooth. Prof Afdhal stressed that the ability to increase platelet levels without a transfusion makes the process for dental extraction much more straightforward. Prof Giannini thought that closed procedures, in which no practical haemostasis can be performed, are where TPO-R agonists can be used most effectively. He also thought that dental procedures for patients on the liver transplant list, where several teeth need to be extracted, benefit from the long duration effect of TPO-R agonists. He also agreed that TPO-R agonists allow repeated procedures to be scheduled, without the need for repeated platelet transfusions.
Closing Remarks
Prof Peck-Radosavljevic concluded that the short duration of an increase, if any at all, in platelet count with platelet transfusions is concerning. With the new TPO-R agonist agents, platelet counts are reliably increased and these agents are extremely useful as the procedure can be pre-planned. Platelet transfusions are still commonly performed, without considering the superiority of the TPO-R agonist alternatives. Increased awareness of the benefits of TPO-R agonist therapy will increase patient safety during elective invasive procedures.








