CLINICAL CASE
An 82-year-old female patient with a history of chronic heart failure New York Heart Association (NYHA) Class II–III due to aortic stenosis was evaluated by progression of their symptoms. An echocardiogram (ECG) confirmed a severe degenerative aortic stenosis with normal left ventricle ejection fraction. The case was discussed by the heart team, and, considering the patient’s age and fragile condition, transcatheter aortic valve implantation (TAVI) was the first option; the patient had a Society of Thoracic Surgeons (STS) risk score of 2.11%. Coronary angiogram showed no significant coronary stenosis and preprocedural computed tomography (CT) revealed an aortic annulus with a mean diameter of 22.3 mm, sinus of valsalva diameter of around 25 mm, a left coronary artery ostium with a height of 4.6 mm, and a right coronary ostium of 9.3 mm. Due to the extremely low height of the coronary arteries and the subsequent high risk of coronary occlusion, the patient was offered a surgical replacement that was rejected. The authors then planned transfemoral-TAVI under general anaesthesia, selecting a valve that would allow them to reposition, recapture, and, eventually, easily access the ostia of the coronary arteries if necessary. The valve used was a Portico No. 23 mm (Abbott Laboratories, Abbott Park, Illinois, USA) self-expanding and repositionable valve. Firstly, the left main coronary artery (LMCA) was protected using a 3.5 JL guiding catheter, a guide wire, and a stent; this was to be used as a rescue technique in case of LMCA occlusion, which seemed to be the most likely adverse event. Secondly, the authors performed an aortic valvuloplasty with an 18 mm Cordis balloon (Cordis, A Cardinal Health Company, Milpitas, California, USA), during which they injected contrast that confirmed flow to both coronaries. Finally, while maintaining the guiding catheter to protect the LMCA, the authors began a controlled release of the valve until complete expansion without incident, certifying normal flow through both coronary arteries with the absence of aortic regurgitation at the end of the procedure. They then easily removed the left main protection system (Figure 1).
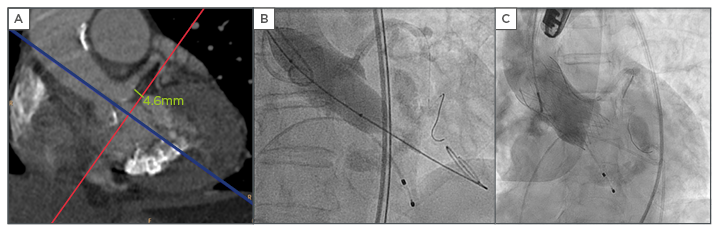
Figure 1: A) Multidetector computed tomography (CT) scan showing the LMCA height of 4.6 mm; B) certifying normal flow through the coronary arteries during balloon inflation; C) controlled valve release and LMCA protection system.
LMCA: left main coronary artery.
DISCUSSION
TAVI is a feasible technique in patients with severe aortic stenosis and low coronary artery height, but there are several safety measures that should be considered: root angiography at the time of balloon valvuloplasty, the use of repositionable and recapturable valves, careful positioning of the transcatheter valve, and placement of a guidewire (with or without a stent) in the coronary ostium at the time of valve deployment.1-3








