Abstract
In patients with angina symptoms but with no coronary artery disease, as revealed by normal or near-normal coronary angiogram, a potential diagnosis of microvascular angina (MVA) might be considered.
This review examines the evidence on long-term prognosis, state-of-the-art assessment and treatment strategies, and the overwhelming need for standardisation of diagnostic pathways in this patient population. The rising clinical relevance of MVA is explored along with how the absence of obstructive coronary artery disease on coronary angiography may not be a guarantee of benign prognosis in this patient subgroup.
A definitive diagnosis of MVA requires evidence of coronary microvascular dysfunction found in up to 60% of patients with symptoms or signs of myocardial ischaemia and no obstructive coronary artery disease. Sex differences affect immune responses associated with hormonal, genetic, and environmental factors, and identification of patients susceptible to microvascular dysfunction ultimately requires the examination of the functional capacity of microvasculature for the proper diagnosis of MVA.
Studies of novel therapies are now more widely available, the positive results of which will encourage more extensive studies in the future. Currently, the evidence base seems to support a stratified approach with medication therapy tailored to the findings of the assessment of the microcirculation.
![]()
Erratum: Microvascular Angina: Diagnosis, Assessment, and Treatment
Authors: Angela H.E.M. Maas, Dejan Milasinovic, Colin Berry, Javier Escaned
Original citation: EMJ Int Cardiol. 2019;7[Suppl 1]:2-17.
Date correction published: 25.9.2019
The article by Maas et al. in Suppl 1 of EMJ Interventional Cardiology 7.1 (pages 2-17) was originally published on 2.9.2019. Since then an erratum has been made. In Figure 1 the label “≥90% diameter reduction” originally read “>90% diameter reduction” incorrectly. This has now been updated.
The EMJ apologises for the error and any inconvenience caused.
![]()
PART 1: DESCRIBING THE PROBLEM OF NO OBSTRUCTIVE CORONARY ARTERY DISEASE IN PATIENTS WITH ANGINA SYMPTOMS FOR WHICH THE CAUSE NEEDS TO BE INVESTIGATED
Background
Almost half of patients with symptoms of angina pectoris have no obstructive coronary artery disease (NOCAD) revealed by coronary angiography. Many of these patients have a normal or near normal coronary angiogram. This conundrum presents a diagnostic challenge to clinicians, leading to uncertainties regarding appropriate treatment and patient information.1 The term microvascular angina (MVA), which suggests that coronary microvascular dysfunction (CMD) is the mechanism of angina in patients, was introduced to provide a clinical definition for the significant proportion of patients who present with symptoms and/or signs of myocardial ischaemia yet without obstructive coronary artery disease ([CAD] in most studies defined as absence of epicardial coronary stenosis >50%) at coronary angiography. Around 40–50% of patients fall into this category,2,3 with a high frequency of angina without constructive CAD (ANOCA) found more commonly in females compared to males (10%).1,4 It is estimated that in the USA and Europe around 3–4 million individuals who present with symptoms of myocardial ischaemia have no obstructive CAD,5,6 and the disease is often unrecognised and undertreated.7 MVA was originally described as a cause of chest pain in patients with normal coronary arteries on angiography,8 which may include abnormal functioning of endothelial and smooth muscle cells that line the heart arteries. MVA associates with vascular risk factors and atherosclerosis is a common finding with coronary angiography.
For this subgroup of patients with ANOCA, CMD may result in transient myocardial ischaemia and a reduced coronary flow reserve (CFR).9 As microvascular dysfunction may also be present in conjunction with obstructive CAD and/or myocardial diseases, the clinical entity of MVA is based on what has historically been known as Type I CMD,9 i.e., microvascular dysfunction in the absence of CAD and myocardial diseases.
Patients with obstructive CAD (mostly defined as ≥50% luminal stenosis and/or fractional flow reserve [FFR] ≤0.80) have established diagnostic pathways with wide availability and access to evidence-based treatment. However, patients with MVA may not only receive an incorrect diagnosis, but until recently, were also considered to be at low risk of cardiovascular events. This assumption is based on little solid evidence to date, with more recent data showing increased risk of cardiovascular events in at least a subpopulation of patients without obstructive CAD on angiography.10,11 Therefore, the emphasis now is to facilitate the adoption of clinically-available diagnostic methods to rule-in or rule-out the diagnosis of microvascular and vasospastic angina, complementing coronary angiography.1 Stratified medicine involves the identification of patient subgroups within an undifferentiated population. The subgroups, defined as endotypes, are distinguished by specific disease mechanisms amenable to targeted therapy.
Consolidation of the nomenclature for chronic (stable) coronary syndromes will help to clarify some of the uncertainties and knowledge gaps caused by dysfunction of the coronary microcirculation.10 Historically, the poorly understood enigma of anginal symptoms and/or signs of myocardial ischaemia coupled with no obstructive CAD on angiography used to be referred to as Syndrome X. This term was unhelpful, especially to affected patients (commonly women), who were prone to misdiagnosis, under-recognition, and sub-optimal management. The contemporary literature has evolved to champion more specific terms such as ANOCA, ischaemia and no obstructive CAD (INOCA), MVA, and vasospastic angina, which should not be used interchangeably.12 Although slightly different from a diagnostic point of view, it is also worth noting myocardial infarction (MI) with nonobstructive coronary arteries in which the presence of acute MI criteria with the absence of obstructive CAD also presents clinicians with similar diagnostic challenges and patient management.13
This multitude of definitions mirrors the heterogeneity of clinical presentations, and at the same time seems to have hampered a systematic approach in terms of both clinical practice and research. The Coronary Vasomotion Disorders International Study (COVADIS) group proposed a standardised set of criteria for the diagnosis of MVA consisting of angina symptoms, absence of obstructive CAD (i.e., <50% stenosis or FFR >0.80), objective evidence of myocardial ischaemia on stress testing, and functional impairment of coronary microvasculature. Per expert consensus, all four criteria must be present for the diagnosis of definitive MVA.14 On the other hand, MVA is considered if symptoms of INOCA on angiography are present, in addition to either objective evidence of myocardial ischaemia or microvascular impairment.14
The primary focus of this article is to highlight to practicing clinicians the clinical relevance of identifying (rule-in, rule-out) MVA in patients with ANOCA. The authors review the evidence on long-term prognosis, the standardisation of diagnostic pathways including the assessment of coronary microcirculation, and the evolving treatment strategies.
Clinical Relevance
The rising clinical relevance of MVA is mainly explained by two contemporary findings: in more than a half of patients investigated for angina symptoms there is no evidence of obstructive CAD on angiography,2,3 and the absence of obstructive CAD on coronary angiography may not be a guarantee of benign prognosis in this patient subgroup.15,16
Initial reports of an impaired prognosis in patients without obstructive CAD on angiography originate from The Women’s Ischaemia Syndrome Evaluation (WISE) study, which showed that women who presented with signs and symptoms of myocardial ischaemia, and who were confirmed to have no obstructive CAD, had an increased risk of major adverse cardiovascular events (MACE), including death, MI, stroke, and hospitalisation for heart failure.17 Of note, this group was also more likely to experience a delay in diagnosis for ischaemic heart disease (IHD).18 A 10-year follow-up data of the WISE study seemed to challenge the notion of an entirely benign prognosis, because the rates of cardiac mortality were 11% and 6% in women with nonobstructive CAD and angiographically normal coronary arteries, respectively.15
These findings were confirmed for both sexes in a large observational trial including 11,223 patients undergoing coronary angiography based on anginal symptoms and 5,705 reference patients without IHD, which showed an increase in 5-year risk of MACE and all-cause mortality in patients with symptoms of stable angina pectoris but without obstructive CAD, as compared with the reference population.2 Moreover, presence of CMD in patients without significant CAD has been associated with impaired prognosis.2
The main problem associated with a lack of angiographic evidence of stenosis in major coronary arteries is that no systematic diagnosis of patients with angina symptoms is done. In general, a scarcity of statistical data exists for patients with specific microvascular disorders, in direct contrast to the widely available prevalence and incidence data for coronary heart disease. Of the limited studies that sought to evaluate patients with chest pain and normal coronary angiography, most are from small single centres.19 That is not to say that the information gained is not valuable, but to gain a broader epidemiological knowledge further research of the microcirculation is required.20
The adverse prognosis of patients with anginal symptoms and no obstructive CAD has been shown to, at least in part, originate from an increased occurrence of MI21 and development of heart failure, including the variant with preserved ejection fraction (HFpEF). There is also evidence of plaque rupture by intravascular ultrasound assessment in some patients, implying a thrombotic pathophysiology 2,6,22,23
In addition to hard endpoints, these patients tend to experience recurrent chest pain leading to repeated visits to the general practitioner, hospital emergency departments, and rehospitalisation for chest pain and repeated coronary angiography. Understandably, chronic chest pain can impair mental health and lead to long-term psychological morbidity. Taken together, these patients experience a reduced quality of life leading to an increased burden placed on healthcare services for repeat assessments and procedures.24
As previously explained, definitive diagnosis of MVA requires the evidence of CMD, which has been shown to be present in up to 60% of patients with symptoms or signs of myocardial INOCA.25 Moreover, presence of CMD in patients without significant CAD has been associated with impaired prognosis.11
In summary, when evaluating the risk of cardiovascular adverse events in patients with MVA in everyday clinical practice, it is important to note that, so far, available evidence stems mainly from heterogenous observational data without a uniform definition of MVA. In practical terms, this means that most of the patients had anginal symptoms without obstructive CAD on angiography, but the evidence of myocardial ischaemia and microvascular functional assessment were not always present. This may need to be kept in mind when interpreting hitherto published data, considering that most of the studies demonstrated incremental elevation of cardiovascular risk in patients with normal coronary arteries on angiography versus nonobstructive CAD versus obstructive CAD. However, as microvascular dysfunction may be found in patients with angiographically normal coronary arteries and nonobstructive CAD, its presence may be the key determinant of prognosis in the overall population of patients with angina but without obstructive CAD, thus making the assessment of microcirculation a key diagnostic step in these patients.
Patient Identification
Vascular risk factors, such as hypertension, cigarette smoking, and obesity, associate with CMD.26,27 On the other hand, affected patients, particularly women, may lack vascular risk factors. In some women, oestrogen withdrawal in relation to the menstrual cycle and menopause is implicated. Endothelial dysfunction is strongly linked with known traditional risk factors and lifestyle factors. Reactive oxygen species released in the body from smoking are known to cause endothelial damage and inflammation; an important twin study provided evidence of a lower CFR in smokers than nonsmokers (2.25 versus 2.75, respectively; p=0.03).26 Impaired CFR is also seen in patients with diabetes, hypercholesterolaemia, and other common risk factors.28 Another important risk factor is stress; men and women handle stress differently and stress-related factors appear to be more related to nonclassical manifestations of IHD, such as MVA compared with obstructive CAD.29
Hypertension,30 diabetes,31-33 and low-density lipoprotein34 are associated with abnormalities of the coronary circulation and impaired CFR. In nondiabetics, insulin resistance is associated with CMD.33
Clinicians should consider a diagnosis of MVA when presented with de novo or recurrent angina in a patient without obstructive CAD on angiography or on computed tomographic angiography (CTA). The noninvasive management of patients is evolving and CT coronary angiography (CTCA) is increasingly adopted in preference to noninvasive stress testing. CTCA presents a conundrum. On the one hand, it helps to clarify the diagnosis of angina due to obstructive CAD, and prognosis in relation to coronary heart disease may be improved. On the other hand, a clinical strategy of CTCA does not reduce referral rates for invasive angiography, and anginal symptoms and quality of life are impaired. The CorCTCA trial is prospectively evaluating the prevalence and clinical significance of MVA and vasospastic angina in patients with angina and no obstructive CAD.35
Referral for invasive management based on findings from CTCA, as opposed to functional testing, presents the invasive cardiologist with new challenges because evidence of ischaemia will typically be lacking. In this case, assessment of lesion-level ischaemia by measurement of FFR or a nonhyperaemic pressure index should be considered and when flow-limiting CAD is ruled out, follow-on measurement of microvascular function, such as with CFR and the index of microcirculation resistance (IMR), should be considered.
The diagnosis of MVA includes anginal symptoms, myocardial ischaemia, and microcirculatory impairment.14 If CTA has been performed in preference to a functional testing then information on myocardial ischaemia may not be available. This gap may add to the diagnostic dilemma faced by clinicians and their patients. In daily clinical practice, there are several indicators that may suggest the presence of MVA.
MVA is associated with episodes of chest pain usually initiated by conditions that require an increased myocardial oxygen demand, such as physical effort or stress. In addition, most patients also have symptoms at rest, in the evening, or early in the morning. Duration of symptoms varies over time and many patients feel extremely tired, especially after a busy day. There may be relief with short-acting nitrates, but not at all times.36
On noninvasive stress testing, patients with MVA may show atypical abnormalities. Compared with patients with obstructive CAD, MVA patients tend to exhibit longer-lasting exercise and/or stress-induced angina which may take >10–15 minutes to resolve after exercise ends. They may also have limited or no response to short-acting nitrates, and left ventricular contractile abnormalities are more likely to be absent from the stress ECG.37
Sex Differences
MVA associates with oestrogen withdrawal after the menopause. This is especially the case in women with vascular risk factors including hypertension,30 diabetes,31 dyslipidaemia,34 and insulin resistance.38 Oestrogen deficiency has long been offered as a reason why the majority of women with MVA with impaired endothelial function are postmenopausal, but it is unknown whether the beneficial effects of oestrogen are endothelium-dependent or related to direct vasodilating effects on the coronary arteries.39-41 Indeed, WISE data do not support such a role for oestrogen deficiency.42
Immune response studies report hormonal, genetic, and environmental factors that affect the immune system, so any focus on inflammation needs to take existing sex differences into account because women and men differ in their levels of susceptibility to inflammatory and autoimmune diseases.29 Sex differences can determine an individual’s immune response to autoimmune diseases and consequent chronic inflammation, such as systemic lupus erythematosus and rheumatoid arthritis,43-46 with inflammation directly linked to CMD in patients with MVA who display no conventional risk factors for CAD; this may be key in the pathogenesis of myocardial ischaemia in this patient population.28 There are also sex differences found when investigating invasive measures of CMD; CFR is higher in men than women, while IMR, a more direct measure, remains constant between both sexes.47 More recently, microvascular function was linked with cardiovascular outcomes in patients with end-stage renal disease, with consequent increased hospitalisation rates for this subgroup.48
In terms of prognosis, presence of nonobstructive CAD in women has been associated with a twice higher risk of death or MI as compared with normal coronary arteries on angiography.49 A pooled analysis including 11 clinical trials showed that 30-day mortality was higher in women than in men although this number decreased on adjusting for baseline differences. Namely, female cohort groups tended to be older with more comorbidities, whereas more males were smokers with a history of MI or bypass surgery.50 In addition, older age, range of comorbidities, and underuse of revascularisation procedures in women have all been suggested as reasons to explain the mortality difference between sexes, although there is no conclusive rationalisation.51
Furthermore, an economic burden also exists wherein women evaluated for signs and symptoms of myocardial ischaemia due to CMD may be underdiagnosed and undertreated, the knock-on effect of which results in increased hospital admission rates and repeat coronary angiographies.7,52,53 As CMD occurs in approximately 50–60% of women with chest pain and no obstructive CAD, researchers considered the risk factors that might determine CMD in this subgroup, and studied a group of female patients (n=159) with a mean age of 52.9 years. However, the study showed that the existence of CMD in this group could not be predicted alone by risk factors for atherosclerosis and hormone levels.53
Therapeutic options for post-menopausal women with MVA include the standard therapeutic approaches of pharmacological and nonpharmacological measures. Cardiac rehabilitation may be particularly beneficial.38 There is a limited evidence base for hormone replacement therapy. Given the association with the risk of breast cancer and atherothrombotic events, hormone replacement therapy should be considered on an individualised basis with specialist input.
Taken together, clinical indicators, including traditional risk factors, female sex, and post-menopause, may help with identification of patients susceptible to microvascular dysfunction, but ultimately the interrogation of the functional capacity of microvasculature is required for the proper diagnosis of MVA. Here, a stepwise approach and evaluation guide to the symptomatic patient with no obstructive CAD provides the basis of assessment and the order in which to conduct microcirculatory assessment techniques (Figure 1).46,54-56
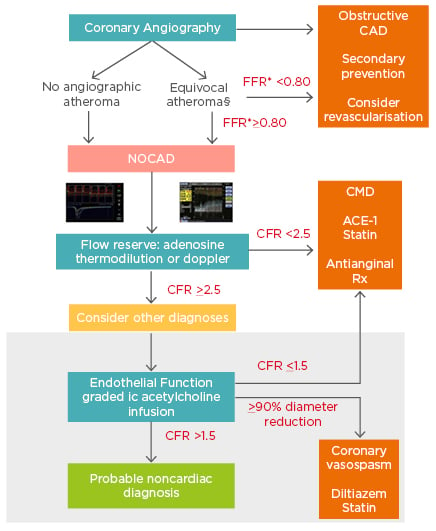
Figure 1: Comprehensive assessment of NOCAD during the time of angiography.
* Evaluation of atheromatous disease can be carried out using resting or submaximal hyperaemic indices based on local practice. §Patients with visible atheroma should be commenced on secondary preventative therapy regardless of final diagnosis. The white area describes tests available on an ad-hoc basis in all catheter laboratories, whereas the grey shaded area describes acetylcholine testing that can currently only be performed on a named-patient basis clinically, or within the context of dedicated research protocols, limiting its widespread ad-hoc use.
CAD: coronary artery disease; CFR: coronary flow reserve; CMD: coronary microvascular dysfunction; FFR: fractional flow reserve; NOCAD: nonobstructive coronary artery disease.
Reproduced from Rahman et al.56
PART 2: ASSESSING MICROCIRCULATION AS A POSSIBLE CAUSE OF ANGINA IN PATIENTS WITH NO OBSTRUCTIVE CORONARY ARTERY DISEASE AND DESCRIPTION OF AVAILABLE TESTS TO ASSESS THE STATUS OF MICROCIRCULATION
Assessment
The mechanisms of angina in patients who present with angina and normal coronary arteries on angiography may range from severe vasospasm to atypical chest pain.57 To properly characterise this heterogeneous patient population, the assessment of microcirculatory function appears to be the key diagnostic tool. Exemplary of the clinical dilemma and uncertainty with respect to the diagnosis of microcirculatory impairment, the WISE study also highlights the ongoing challenge to clinicians for accurate diagnostic testing of women with suspected IHD, regarding the influence of sex hormones on symptoms and response.7 Although limitations to this study include selection bias (all patients were women), the adverse outcomes reported have since been reproduced in several large, population-based registries which also extend to male patients.2,21,23 A diagnostic algorithm is hereby provided to increase awareness of the entire perspective (Figure 2).12
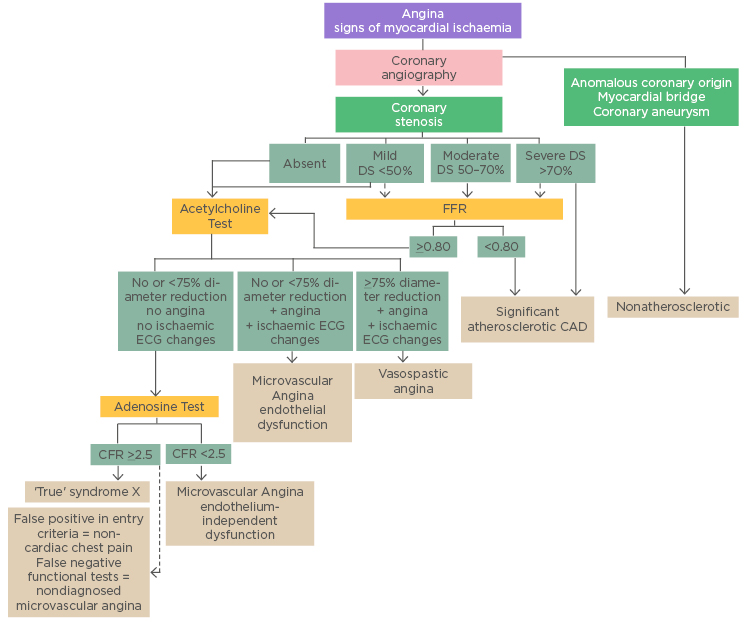
Figure 2: Diagnostic algorithm in patients with angina symptoms and suspected coronary artery disease/dysfunction.
CAD: coronary artery disease; DS: diameter stenosis; FFR: fractional flow reserve.
Copied from Shaw et al.5
The Appendix to the European Society of Cardiology (ESC) guidelines on the management of stable CAD provides specific guidelines on recommended therapy for microvascular and vasospastic angina.35 It should be noted that these guidelines are due to be updated in 2019. As described earlier, COVADIS has called for standardisation of diagnostic criteria for ischaemic symptoms due to CMD for patients who present with chest pain consistent with MVA;14 importantly, this key publication for investigative diagnosis of MVA outlines the agreed criteria from the COVADIS Summits of 2014 and 2015.
How to Start Microcirculation Assessment
For patients presenting to the cath lab, a variety of methods can identify CMD in the presence of normal coronary arteries. Currently, measuring microvascular resistance is used to assess the microcirculation, although results of a new study support vascular conductance as a novel technique to identify patients with myocardial ischaemia; in the latter, epicardial and microvascular domains are assessed separately.58 State of the art in microcirculation assessment includes the following invasive techniques (Table 1).59,60
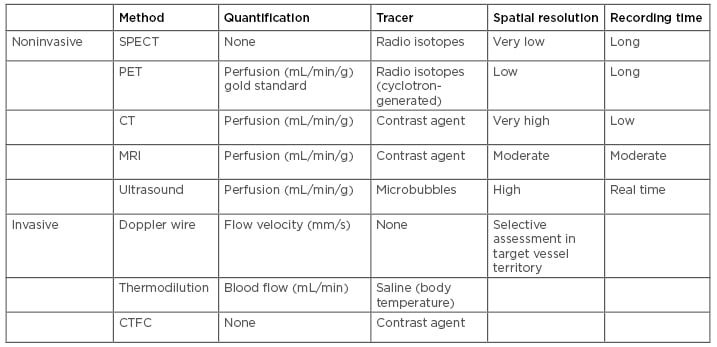
Table 1: Techniques for cardiac assessment of microvascular function.
Reproduced from Frishman.60
Coronary Angiography
This invasive procedure, known as a heart (cardiac) catheterisation procedure, detects an epicardial obstruction of blood flow to the heart. Of note is that with CTA, imaging obstructive CAD can also be excluded and noncalcified plaques can be made more visible than with angiography. However, regarding the microvasculature, both techniques provide limited qualitative and subjective information for diagnostic purposes.61
Coronary Flow Reserve
The CFR reflects the amount of additional blood flow available to the heart above baseline blood flow, and the capacity of the coronary circulation to increase flow following increased myocardial metabolic demands. The CFR is defined as a ratio (CFR at stress: CFR at rest) and can be derived by several methods, including myocardial contrast echocardiography (MCE-derived CFR), PET, and single-photon emission computed tomography (SPECT).62,63 In addition, pressure-derived methods evaluate flow reserve through pressure-tipped catheters which provide an estimate of coronary FFR and myocardial FFR; the fractional collateral flow is the difference between the two.64 Pressure wire-derived CFR is a safe procedure in experienced physicians to determine microvascular resistance; however, drawbacks include a lack of reproducibility and the true CFR being potentially underestimated. Measurements are not specific for the microvasculature.61
Index of Microcirculation Resistance
The IMR is a thermodilution technique that combines a coronary pressure wire and commercially available software to perform a quantitative assessment of microvascular resistance. Measurement is made during maximal hyperaemia and the IMR reflects the functional status of the microcirculation. The technique is reproducible, quantitative, and specific for the microvasculature, but requires experienced operators.61,64-69
First validated some 16 years ago in an animal model, the IMR is derived from Ohm’s Law for fluid flow; microvascular resistance is calculated from the change (∆) in pressure across the microcirculation (distal coronary pressure [Pd] minus venous pressure [Pv] divided by flow). Coronary flow is an estimate of the inverse of the mean transit time (Tmn); Pv is typically negligible compared with Pd and may not feature in the equation.38
Resistance = ∆ Pressure / Flow
∆ Pressure = Pd – Pv Flow (1 / Tmn)
IMR = Pd x Tmn at maximal hyperaemia (IMR <20 is in the normal range)
Resistance Reserve Ratio
Resistance reserve ratio is the ratio of basal resistance:IMR, and a measure of the vasodilatory capacity of the microcirculation.
Fractional Flow Reserve
FFR is the gold standard technique for the assessment of the haemodynamic significance of an epicardial stenosis; however, clinical decisions are directly affected by the accuracy of measurements, interpretation may be problematic, and it does not assess the microcirculation.67-70
Coronary Blood Flow and Epicardial Coronary Artery Diameter
Measurements are performed using endothelium-dependent probes (ACh, bradykinin, substance-P, L-NMMA, shear-stress) and endothelium-independent probes (adenosine or sodium nitroprusside).6 Procedure-related adverse events are reported as <1% compared with coronary angiography; however, standardised protocols are limited, safety data in women is minimal,71 and catheterisation lab time may be an issue.
Techniques used to assess CMD are based upon patient level of risk and vary between centres according to availability of techniques and expertise. For an individual at low-to-intermediate risk, a stress test with ECG will determine exercise capacity and tolerance. One limitation of this approach is the potential for false positive test results.25,72 Patients considered as intermediate-to-high risk with abnormal ECG may undergo myocardial perfusion imaging (MPI; nuclear stress testing through SPECT or PET,73 or alternatively, stress echocardiography with cardiac MRI or CT-angiography. For higher-risk patients with an abnormal MPI study, the angiogram is paired with an invasive test to determine CFR.74 Another approach is simply to invoke tests of coronary vascular function in the catheter laboratory when ANOCA is suspected on clinical grounds.75
Although the focus of this article is on the invasive aspects of assessment, it is pertinent to highlight some novel noninvasive techniques for assessing patients with angina. Recent studies include: the use of cardiac magnetic resonance for detection of ischaemia due to obstructive CAD in which the authors report on pixel mapping of myocardial blood flow estimated from stress perfusion;67-69 assessment of glycocalyx-mediated microvascular function using sublingual microscopy imaging and investigation of the myocardial perfusion reserve;76 measuring the flow in the left anterior descending (LAD) using transthoracic doppler echocardiography, in which CFR is measured as the ratio of hyperaemic diastolic peak flow velocity during adenosine induced maximal vasodilation to basal flow velocity; and stress perfusion echocardiography, another noninvasive, real-time imaging modality.77 However, it should be noted that the use of this technique can be operator dependent, accompanied by operational difficulties and potentially disappointing results, as well as time consuming. Indeed, it is important to note that the reliability of all these measurements are investigator and patient dependent.75
Addressing a patient-centred approach to care, a recently published randomised CorMicA trial78 of outpatients undergoing elective angiography as standard of care for the investigation of angina, received a stratified medical therapy based on the results of an interventional diagnostic procedure (IDP), which included intravenous infusion of adenosine (140 µg/kg/min) to assess CFR (abnormal <2.0), IMR (abnormal ≥25), and FFR (abnormal ≤0.80), as well as administration of acetylcholine to provoke vasospasm. This first randomised sham-controlled trial of coronary function testing in patients with INOCA reported patient benefits of improved angina severity and increased quality of life at 6 months. The results of this study highlighted the limitations of invasive coronary angiography to identify patients with MVA and/or vasospastic angina.
PART 3: TREATMENT AND THERAPIES
Current Treatments and Prevention
Risk factor management is central to any treatment approach for patients with chest pain and no obstructive CAD, and this begins with lifestyle counselling. Studies show that diet quality is instrumental in reducing CVD incidence and events, and especially in patients with comorbidities such as diabetes.79 Results of a prospective cohort study of 31,546 women and men revealed that our understanding of what constitutes healthy eating may be in question, as dietary constituents and nutrients are not consumed in isolation, e.g., salt, sugar. Results from two randomised trials, the Ongoing Telmisartan Alone and in Combination With Ramipril End Point Trial (ONTARGET) and the Telmisartan Randomised Assessment Study in ACE-I Intolerant Subjects With Cardiovascular Disease (TRANSEND), confirm that when the overall diet quality improves, the risk of recurrent CVD events is reduced beyond that of drug therapy alone.79
Comorbidities need to be aggressively managed through a programme of exercise, weight loss (natural and surgical), smoking cessation, and stress management.78 Blood pressure, as well as diabetes and related metabolic disorders, all need to be controlled, with lipids managed through improved diet as discussed.80 Other nonpharmacological therapies shown to increase the health benefits of patients include cognitive behavioural therapy81 and meditation,6 which may be helpful in stress management.
First-Line Therapy
Evaluation of treatment strategies for patients with MVA is challenging because of the absence of a standardised definition and diagnostic criteria. Current recommendations are for traditional antianginal therapies, such as β-adrenergic receptor blockers (beta-blockers), calcium channel blockers, and short-acting nitroglycerine. The prophylactic use of long-acting nitrates may have an adverse effect on ischaemia due to the development of tolerance which results in an overall reduction of the antianginal effects.82
Beta-blockers can reduce the occurrence of angina episodes, improve ischaemic threshold, and even improve endothelial function in some patients through a possible antioxidant effect, although this remains to be proven widely in a clinical setting.83 The choice of drug class will be dependent upon patient tolerance and preference, contraindications, and the presence of comorbidities,84 and are not recommended for patients with vasospastic angina. Also, abrupt withdrawal may result in rebound myocardial ischaemia.
Calcium channel blockers reduce afterload and increase the myocardial blood flow, while reducing heart rate and contractility. Systemic and coronary vasodilation is achieved through interaction with L-type Ca2+ receptors. It is preferable to use long-acting preparations and contraindications for nondihydropyridine calcium channel blockers are similar to those for beta-blockers.59
Organic nitrates can reduce myocardial oxygen demands while maintaining or increasing coronary artery flow, and are longstanding treatment for angina pectoris.60 Their safety profile allows them to be used with both beta-adrenergic or calcium-channel blockers, although several studies report no benefit in patients with chest pain and no obstructive CAD.85,86 Treatment with oral nitrates necessitates an individualised approach. Usually angina symptoms will improve with oral nitrate therapy, especially in patients who benefit from sublingual nitrates. In some patients, angina may deteriorate, presumably due to a steal phenomenon by vasodilatation of collateral vessels. Some patients may not tolerate statins because of side effects. Statins, angiotensin-converting enzyme inhibitors (ACE-I), and low-dose aspirin are also current pharmacology for CMD with the aim of treating microvascular endothelial dysfunction.6,78 Patients with exercise-induced ischaemia and flow-mediated dilation respond to statin therapy, and the observed beneficial effects are considered to be attributable to improved endothelial function.87 ACE-I medication is effective in patients with MVA with improvement to CFR in this patient population.88 The WISE double-blind randomised study of women with symptoms of cardiac ischaemia, nonobstructive coronary arteries, and MVD, revealed improved microvascular function in a substudy of 78 women.89 If patients are intolerant of ACE-I, angiotensin receptor blockers are an alternative, although there are no data for how effective this therapy may be for CMD patient subgroups. Additionally, for patients with insulin resistance, metformin administration is effective in increasing microvascular function.90 Imipramine, a tricyclic medication, also improved the symptoms of patients with chest pain and no obstructive CAD; this was thought to be due to a visceral analgesic effect.91 Aspirin may be beneficial in patients with CAD but when CAD is excluded there is no evidence to support treatment with aspirin. In this case, the risk of bleeding events outweighs any theoretical benefits.
Novel Therapies and Technologies: The Need for Personalised Medicine
Standard pharmacological treatment for myocardial ischaemia is often unsuccessful in patients with chest pain and no obstructive CAD and may be attributable the different causes of angina in this patient subgroup. With traditional antianginal drugs only proving effective in around half of patients with MVA, there is a growing need for more research to investigate novel therapies.92 A personalised approach is needed with follow-up of individual patients to tailor therapy according to symptom relief and side-effects.
Ranolazine studies have shown favourable outcomes for improved symptoms of angina in patients with Type 1 coronary MVD (CMVD) and impaired CFR, although the mechanism remains unclear.93 A smaller study investigated the impact of ranolazine on Type 1 CMVD and reported favourable IMR changes and improved symptoms of angina in a small patient cohort (n=7).94 Patient benefits with ranolazine in studies focussed to those patients with a reduced CFR are also reported, but in this particular study IMR was not measured.95 Recent expert reviews have provided a comprehensive summary of the clinical trials and treatment options for MVD.96
Stratified medicine holds promise to guided patient management in daily practice. In the CorMicA trial, therapy was stratified based on the IDP performed as an adjunct to invasive angiography in patients with ANOCA (stenosis <50.00% and FFR >0.80).77 These patients were divided into three diagnostic groups: 1) vasospastic angina that was documented by an acetylcholine-provoked epicardial artery spasm that resolved with intracoronary nitroglycerine; 2) MVA that was characterised by CFR <2.0 and/or IMR ≥25.0 and/or microvascular spasm to acetylcholine; and 3) noncardiac chest pain with CFR >2.0 and IMR <25.0 and no vasospasm to acetylcholine. For patients with vasospastic angina, smoking cessation, calcium antagonists, and long-acting nitrates, as well as lifestyle changes were advised, whereas patients with the diagnosis of MVA received a beta-blocker (e.g., nebivolol), ACE inhibitors, and consideration for statins. These patients were also advised in terms of lifestyle change (weight loss, smoking cessation, cardiac rehabilitation programme). For patients with a diagnosis of noncardiac chest pain, antianginal therapy was stopped, and further noncardiac investigation considered. Compared with controls in whom IDP had also been performed (the results were not disclosed to the treating cardiologists, who based their decisions solely on angiography and other available medical information, but not IDP), outcomes at 6 months showed a clear improvement in angina symptoms and quality of life in the intervention arm. These patient benefits are attributed to tailored therapy, better engagement, and better-informed patients.77
Current and investigational drugs with endothelial protective effects target the specific mechanisms underlying endothelial dysfunction, and novel cardiovascular medications are more effective in the protection of the endothelium compared with the more variable effect on endothelium function of existing therapies.97,98
Long-term beneficial effects of ACE inhibitors in retaining vascular integrity may be linked with the protective action of bradykinin as an investigational drug against ROS and toxin-induced microvascular endothelial cell death. However, the clinical use of bradykinin remains contentious due to an association with inflammation and cancers.99 A beneficial effect on endothelial function in hypertensive patients with obesity has been demonstrated using a combined therapy of carvedilol (a nonselective ß1 and ß2 antagonist) with an ACE inhibitor,98 although calcium-channel blockers also improve endothelial function in normotensive hypercholesterolaemic patients with no adverse effect on blood pressure or lipid levels. In addition to ACE inhibitors, angiotensin receptor blockers also improve endothelium-dependent vasodilation, but conflicting results have been obtained in the microcirculation.97
Refractory angina may also be a presenting symptom of microvascular disease, and novel technologies on the market have demonstrated a positive effect on this patient population by reducing the burden of disabling symptoms and improving quality of life. The Reducer is a medical device that creates a focal narrowing in the lumen of the coronary sinus generating a pressure gradient across the device within 4–6 weeks after implantation; at this point tissue ingrowth covers the metal mesh design. Symptoms of angina are alleviated because of improved perfusion achieved through redistribution of blood within the ischaemic subepicardium.100
For individuals with MVA there is no conclusive evidence that supports a specific class of drugs or combined therapy, or therapy and technology, presumably because of the knowledge gap regarding the cause of MVA and the inconsistency of patient response to available drug treatments.101
Case Studies
The first case study describes a 59-year-old male with a 7-year history of exertional chest pain radiating down his left arm and a medical history of Type 2 diabetes mellitus, hypertension, dyslipidaemia, a family history of premature CAD, a previous transient ischaemia attack, and obesity. On initial presentation 7 years ago, a myocardial perfusion scan was reported to have shown inducible ischaemia. The patient then underwent invasive coronary angiography, showing an anomalous circumflex artery, but otherwise unobstructed coronary arteries. His antianginal treatment was stopped by his attending cardiologist. In the last year, the patient presented again to the local cardiology service with worsening symptoms. A CTCA was performed, showing mild plaque disease only. Due to limiting symptoms, the patient underwent a further invasive coronary angiogram and coronary physiological test (Figure 3), revealing impaired coronary microvascular function (CFR of 1.6 and an IMR of 59). The patient was subsequently diagnosed with MVA and started on verapamil.
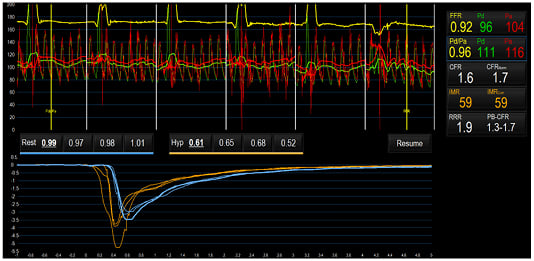
Figure 3: Case study assessment of coronary microvascular function.
Microvascular function measured using a pressure wire coupled with thermodilution, at rest and during maximal hyperaemia, demonstrated impaired coronary microvascular function (CFR 1.6 and IMR 59).
CFR: coronary flow reserve; FFR: fractional flow reserve; Hyp: hyperaemia; IMR: index of microcirculatory resistance; Pa: mean proximal coronary pressure; Pd: mean distal coronary pressure.
The second case study describes a 69-year-old female who started to experience anginal symptoms 8 years earlier. A smoker (15/day) since her teens, she also was afflicted by diabetes and hypertension for >10 years. Her father was diagnosed with acute coronary syndrome at the age of 54 years and her mother suffered a stroke when she was 72 years old. A coronary angiogram was performed when the patient first started to experience symptoms, showing a nonsignificant lesion in the LAD. A year after the patient’s first symptoms, she had a minor stroke and subsequently visited the outpatient clinic over a number of years with stable symptoms of chest pain, both at rest and during exercise. An exercise test was carried out several times, being nonconclusive without many symptoms. At this stage the patient had been unable to stop smoking. Gradually, her symptoms of chest pain and dyspnoea increased over the years, resulting in a recent coronary angiogram with coronary testing. Figure 4 shows her left coronary artery (LCA) with diffuse atherosclerosis, but no significant lesions. FFR was 0.88 in the LAD, CFR was 2.5, and IMR 42. At acetylcholine testing up to 200 µg, no significant changes in the diameter of the epicardial coronary arteries were seen. It was concluded that the patient had diffuse NOCAD with MVD. Treatment continued with aspirin, a statin, β-blocker, and an ACE-I. The patient did not tolerate calcium antagonists well. The patient was also able to stop smoking.
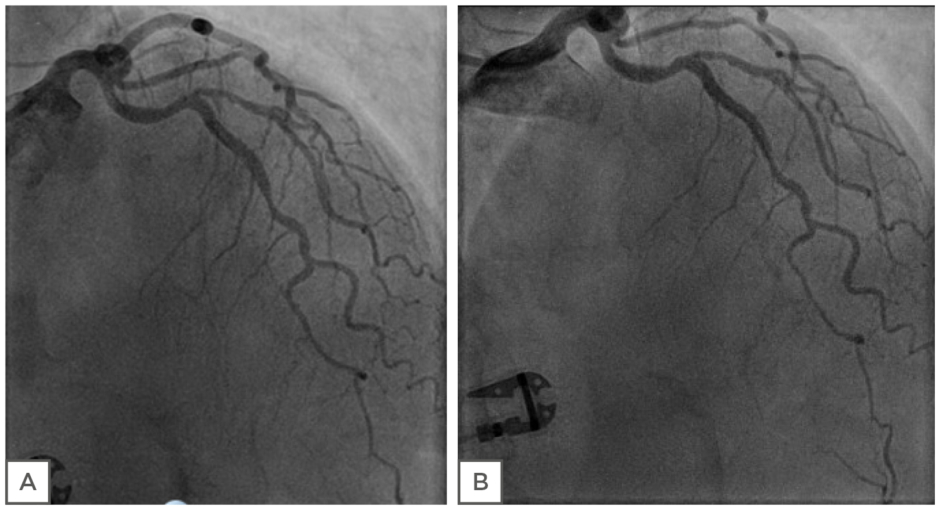
Figure 4: Case study assessment of coronary endothelial function. Left coronary artery (LCA) of patient before (A) and after (B) the infusion of 200 μg ACh. No significant changes in the diameter of epicardial coronary arteries were evident.
CONCLUSION
Angina with NOCAD is a common outcome following coronary angiography. Given the long-term health burden of affected patients, and emerging evidence in favour of stratified therapy, a proactive approach is justified. A variety of mechanisms underpin the origin of chest pain in patients with angina and no obstructive CAD. Novel invasive diagnostic approaches now empower cardiologists to rule-in or rule-out coronary vasomotion disorders in the catheter laboratory. There are several methods by which to evaluate the microcirculation in the catheterisation lab, such as IMR and CFR. Availability of existing qualitative and quantitative techniques help guide the treatment of patients with chest pain and normal arteries on angiography. Results from research into novel therapies is now more widely available as seen from the CorMicA trial and ranolazine studies, the positive results of which will encourage larger studies in the future. Currently, the evidence base seems to support a stratified approach with medication therapy tailored to the findings of the assessment of microcirculation. More attention to this patient population and more rigorous investigation of the issue is urgently needed.








