Abstract
The left main coronary artery originating from the right sinus of Valsalva is a rare congenital anomaly. A 62-year-old male came in with recurring exertional chest discomfort, as observed by the authors. He was referred to the catheterisation laboratory for coronary angiography, which revealed the presence of a left main coronary artery coming from the right coronary sinus. In the proximal portion of the left anterior ascending coronary artery, a critical stenotic lesion was identified. The cardiac CT angiography demonstrated a benign retroaortic course. The lesion was effectively treated utilising three drug-eluting stents.
Key Points
1. A 62-year-old male presented with recurring exertional chest discomfort. Coronary angiography confirmed the existence of a left main coronary artery originating from the right coronary sinus.2. The report details the patient’s clinical presentation, diagnostic workup, treatment strategy, and follow-up. It also emphasises the significance of early detection and therapy of this condition to avoid negative results.
3. Congenital anomalies of the origin and/or course of the coronary arteries are a key differential diagnosis for exertion angina. Multimodal coronary imaging is essential for determining the best course of action.
BACKGROUND
Multiple studies indicate that the incidence of congenital coronary artery anomalies ranges between 0.3–5.6%.1,2 Due to the wide availability of cardiac CT angiography (CCTA), however, a new study estimates the incidence to be 7.9%.3 The most prevalent categorisation scheme is Angelini’s 1999 proposal, which separates anomalous coronary arteries into three primary categories: anomalies of origin and course, anomalies of termination, and anomalies of intrinsic coronary artery architecture.4
Anomalies of origin and course are differences in the location of origin and course of the coronary arteries. They are further categorised into two subtypes: anomalies of opposite sinus of Valsalva (OSV) origin, and anomalies of interarterial course.5
Anomalies of OSV origin are coronary arteries that emerge from the opposite aortic sinus and run between the aorta and the pulmonary artery. This subtype comprises an anomalous LM from the right sinus, an anomalous right coronary artery from the left sinus, and an anomalous left circumflex artery from the right sinus.6
Anomalies of interarterial course are coronary arteries that run between the aorta and the pulmonary artery, regardless of their origin. This subtype consists of anomalous left main coronary artery (LM) from the right sinus, an anomalous right coronary artery from the left sinus, and the aberrant origin of both coronary arteries from the same aortic sinus.6
The abnormality may be classified into five clusters based on the anatomical link between the abnormal vessel, the aorta, and the pulmonary trunk. In a preaortic (interarterial) course, the aberrant vessel travels between the aorta and pulmonary artery. A pre-pulmonic (anterior) anomalous vessel follows the outflow of the right ventricle anteriorly, a subpulmonic (septal) anomalous vessel takes an intramyocardial path before reappearing in the proximal part of the interventricular groove, and a retroaortic (posterior) anomalous vessel travels posteriorly around the aortic root. In a retrocardiac course, an abnormal vessel flows behind the heart, as opposed to in front of it. The first aberrant pattern is regarded as the most threatening, since it raises the risk of sudden cardiac death (SCD) in those individuals.5-10
Anomalies of termination relate to variations in the distribution and termination of the coronary arteries. They are further categorised into ALCAPA and coronary artery fistulas.11 ALCAPA refers to an aberrant left coronary artery that terminates in the pulmonary artery, as opposed to the aorta. This condition, also known as Bland-White-Garland syndrome, has the potential to produce left ventricular dysfunction and heart failure.12
Fistulas of the coronary arteries are aberrant connections between the coronary arteries and other heart chambers or vessels. These connections may result in ischaemia, myocardial infarction, or heart failure by diverting blood away from the myocardium.13
Abnormalities of intrinsic coronary artery anatomy include size, shape, and distribution variations in the coronary arteries. They can be further divided into two subgroups: myocardial bridging and aneurysms in the coronary arteries.11 Myocardial bridging refers to a part of a coronary artery that runs through the myocardium, as opposed to above it. This condition can cause ischaemia during exercise or stress.14-16 Coronary artery aneurysms refer to localised dilations of the coronary arteries that can be congenital or acquired. They are commonly seen in patients with Kawasaki disease.17
Based on the patient’s clinical history, symptoms, and risk factors, the diagnosis of aberrant coronary arteries needs a high level of suspicion. In the majority of instances, imaging techniques like echocardiography, CCTA, cardiac MRI (CMRI), and coronary angiography are used to confirm the diagnosis.18,19
Echocardiography is a non-invasive imaging modality that can be used to detect anomalous coronary arteries, especially when combined with colour Doppler imaging. However, its sensitivity and specificity are limited, and it may not be able to detect all types of anomalous coronary arteries.20
CCTA is a highly sensitive imaging modality that can produce detailed images of the coronary arteries and their course. It is particularly useful for detecting anomalous origins of the coronary arteries from the OSV, or interarterial course, as well as coronary artery fistulas. CCTA can also be used to assess the severity of stenosis or obstruction of the coronary arteries.
CCTA is a very sensitive imaging technique that can map the coronary arteries in great detail. Coronary artery fistulas and abnormal origins of the coronary arteries from the OSV can be detected using this technique, as well as assessment of coronary artery stenosis or occlusion.21
CMRI is a non-invasive imaging technique that creates pictures of the heart’s internal structures using magnetic fields and radio waves. It can be used to detect anomalous coronary arteries, especially when combined with contrast-enhanced angiography. CMRI can also provide information about myocardial perfusion and function.22
Invasive coronary angiography is the diagnostic imaging modality of choice for coronary artery anomalies because of the wealth of information it provides regarding the coronary arteries’ anatomy, course, and distribution. Nonetheless, there is a small possibility of repercussions.23
Coronary artery anomalies can range from being completely incidental to being potentially fatal. This depends on the severity of the anomaly, and how it affects the arteries’ origin, course, and distribution. It is important to take the patient’s clinical condition and symptoms into account when deciding how to treat aberrant coronary arteries. In many cases, no treatment is necessary for asymptomatic people who have benign abnormalities, such as myocardial bridging or minor coronary artery aneurysms.24
A surgical or interventional procedure may be necessary for individuals who are experiencing symptoms, or who have high-risk abnormalities such as ALCAPA, or an interarterial course of the coronary arteries. Improving myocardial perfusion and avoiding ischemia, myocardial infarction, and SCD are therapy goals.23,24
Reimplantation of the anomalous coronary artery into the proper aortic sinus or bypass grafting of the aberrant section may constitute surgical therapy. This method can give a permanent cure and enhance long-term outcomes, but it has a greater risk of complications and a longer recovery time.25-27
Interventional therapy may entail percutaneous coronary intervention (PCI) or stent implantation; this procedure is less invasive than surgery, and can provide rapid symptom alleviation. When contemplating PCI, it is crucial to evaluate the course of these anomalies, and rule out malevolent ones. It may not be appropriate for all types of aberrant coronary arteries, and is linked to an increased risk of restenosis or thrombosis.28,29
The prognosis of aberrant coronary arteries depends on the type and severity of the anomaly, as well as the promptness and adequacy of treatment. Benign anomalies, such as myocardial bridging or small coronary artery aneurysms, have an excellent prognosis, and may not require any intervention.4,24
High-risk anomalies, such as ALCAPA or the interarterial course of the coronary arteries, have a poorer prognosis, and may require prompt intervention to prevent ischaemia, myocardial infarction, or SCD. In general, the long-term results of surgical or interventional therapy are favourable, although they are dependent on the patient’s clinical condition and the efficacy of the surgery.30,31
The authors describe a patient with an abnormal LM originating from the right coronary cusp, who suffered from recurrent chest pain, and his treatment.
CASE PRESENTATION
A 62-year-old male, with a past medical history of sensorineural deafness, hypertension, and Type 2 diabetes on oral hypoglycaemic medications, had reported to the cardiology clinic after several months of chest pain during exertion, which was deemed to be ischaemic in origin. A clinical examination, ECG, and echocardiography were done, showing normal findings.
Despite beginning treatment with anti-ischaemic medication, the patient continued to experience symptoms consistent with Canadian Cardiovascular Society (CCS) Grade II angina pectoris; as a result, the decision was made to perform coronary angiography after obtaining informed consent for the procedure. The right radial approach was used, using a 5F Judkins right coronary catheter.
After engaging the right coronary sinus and contrast injection (Figure 1), a small non-dominant right coronary artery (RCA) was revealed, and the LM was found to arise from the right coronary ostium, which subsequently divided into the left circumflex artery and the diseased left anterior descending artery (LAD).
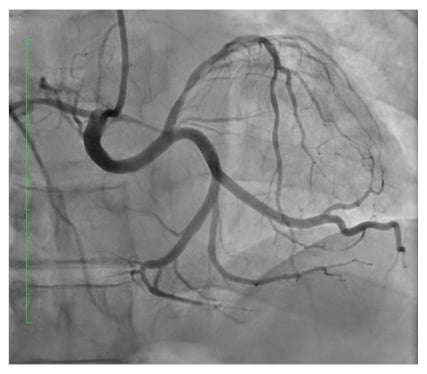
Figure 1: Coronary angiography showing the right and left coronary arteries arising from the right sinus of Valsalva demonstrating significant proximal left anterior descending coronary artery lesion.
MANAGEMENT
Since coronary angiography revealed a significant proximal lesion in the LAD, there was a need to delineate the course of the coronary arteries, for fear of an intra-arterial course with a high risk of SCD. If present, such a malignant course would require a different management strategy. This led to the decision to opt for CCTA. After receiving the patient’s informed consent, a CCTA was performed, which revealed an anomalous LM originating from the right sinus of Valsalva. This aberrant LM followed a retroaortic course, and was the source of both the LAD and the left circumflex artery. The RCA was shown to originate from the more proximal part of the LM (Figure 2).
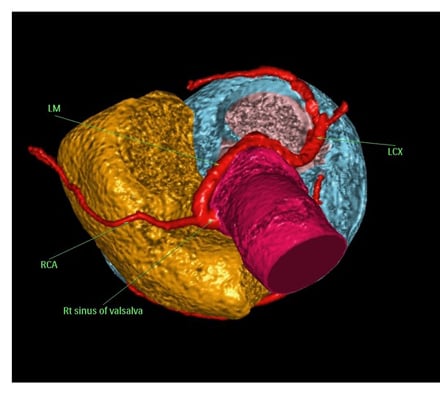
Figure 2: Cardiac CT angiography 3D volume-rendered image demonstrating the retroaortic course of the left main coronary artery from the right sinus of Valsalva after removing the right atrium and left atrium.
The patient underwent PCI to LAD through right radial using a 6F MPA1 guide catheter (Medtronic, Watford, Hertfordshire, UK) and BMW wire (Asahi Intecc, Nagoya, Japan), pre-dilatation with non-compliant balloons of 2.25×15 mm and 2.5×20 mm, followed by deployment of three overlapping drug-eluting stents: XIENCE Skypoint™ Stent (Abbott Vascular, Abbott Park, Illinois, USA) 3.0×28 mm; SYNERGY™ (Boston Scientific, Marlborough, Massachusetts, USA) 2.75×16 mm, and XIENCE Skypoint™ Stent (Abbott Vascular) 2.75×12 mm, followed by post-dilation with stent balloon up to 26 ATM with excellent results achieving TIMI III flow (Figure 3).
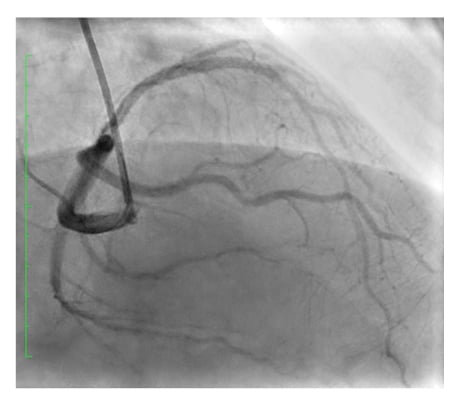
Figure 3: Left anterior descending coronary artery post-stenting with TIMI III flow.
The patient went home on the same day of the procedure on dual antiplatelet, β-blocker, and statins as part of anti-ischaemic treatment, as well as angiotensin receptor blockers for control of hypertension, and empagliflozin with linagliptin for diabetes management.
DISCUSSION
It is challenging to determine the burden of the aberrant origin of the left coronary artery from the right sinus of Valsalva since this abnormality is uncommon, and data from observational studies are very variable.32 However, it accounts for 0.3–1.0% of cases in the dataset of Angelini,1 with a retroaortic course being the most prevalent subtype (prevalence: 0.28%; 95% confidence interval: 0.21–0.35%).7
Anomalous coronary arteries have been reported to be susceptible to atherosclerosis, even at younger ages.33,34 Coronary blood flow would be hampered if aberrant coronary arteries emerged from the other side of the coronary sinus, which is positioned between the pulmonary trunk and the ascending aorta, especially if high-risk criteria are present in the form of a slit-like opening in the proximal vessel morphology, acute angle takeoff, and interarterial course, which was not present in the authors’ patient.35-37
By the time they are 20 years old, many individuals with aberrant LM coming from the opposite right coronary artery and the previously indicated high risk criteria have already passed away, usually during, or shortly after, strenuous exercise, which is characteristic of young, ‘healthy’ athletes.38,39 However, the authors’ patient presented with significant atherosclerotic changes in the LAD originating from the anomalous LM. The retroaortic route of the aberrant coronary artery suggested that the atherosclerotic changes in the coronary arteries were the true culprit in this patient’s case of chest pains, rather than the abnormal artery itself.40-44
Despite advances in interventional technology and procedural improvements, coronary artery anomalies remain a challenge for interventional cardiologists. The use of PCI as a treatment for aberrant coronary arteries appears promising; nevertheless, proper topographical identification of the anomalous vessel’s origin and proximal course is essential.38,45,46
Particularly in the setting of coronary anomalies, the long-term care of patients who have had PCI often entails a variety of interventions, including pharmaceutical therapies, lifestyle changes, and routine follow-up visits.47
Changes in lifestyle, such as exercise, good nutrition, smoking cessation, and a healthy weight, contribute to heart health. Exercise improves quality of life, cardiovascular health, and the risk of future cardiac events. A healthy diet reduces cholesterol, blood pressure, and heart disease. Stopping smoking improves long-term PCI outcomes, since heart disease is a major risk factor. Maintaining a healthy weight improves overall health and reduces the chance of heart issues.48 However, the authors’ patient was not overweight, or a smoker.
Pharmacological treatments to avoid stent thrombosis include PCI followed by dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor. The patient’s health, stent type, and bleeding risk will determine the DAPT length. Statins, β-blockers, and angiotensin–converting enzyme inhibitors may be administered as well. Statins, β-blockers, and angiotensin–converting enzyme inhibitors can decrease cholesterol, blood pressure, and heart rate, respectively.49 The authors’ patient was kept on all these medications and DAPT for 12 months.
Regular follow-up meetings with the healthcare practitioner are crucial for monitoring the patient’s condition, reviewing the treatment plan’s efficacy, and managing problems. Follow-up appointments are usually planned at regular intervals for the first year following the treatment and less often after that. At follow-up sessions, the doctor will examine the patient, check their prescriptions, and prescribe any required tests or imaging investigations. The authors’ patient had his first clinic appointment after 1 month, where he was asymptomatic, and in a compensated state. The authors planned for a 3-month follow-up interval for the first year if he remained asymptomatic.47
Stress testing can measure symptoms, and stratify people who may be at higher risk for cardiac events. Echocardiography may assess heart function, particularly the ejection fraction. CCTA or coronary angiography can be utilised to visualise any possible anomalies in the coronary anatomy and evaluate the patency of any stents or grafts inserted during PCI. These imaging scans can be repeated to evaluate the patient’s status and therapy efficacy.49 However, because the authors’ patient was asymptomatic, no additional evaluation was required.







