Abstract
Percutaneous coronary intervention has been a major interventional medical development of our times, being a life-saving procedure in the setting of acute coronary syndrome, and providing significant improvements in quality of life for patients with chronic coronary syndromes.
Complications of coronary intervention have continued to downtrend, facilitated by improvements in stent and wire technology, the aggressive use of antiplatelets, and the regular use of invasive anatomical and physiological assessments.
The authors present the case of a challenging and rare procedural complication, that of stent delivery shaft fracture requiring emergency snare extraction.
INTRODUCTION
A 65-year-old female was transferred for an emergency coronary angiogram from a peripheral centre with a diagnosis of acute coronary syndrome (ACS).
The patient reported three episodes of severe central chest pain radiating to their left arm, associated with sweats and occurring at rest, with ongoing mild chest pain in the emergency department of the peripheral hospital. Their ECG showed sinus rhythm with a Wellens pattern in V1–V4.
The patient was given aspirin 300 mg and ticagrelor 180 mg, and transferred for invasive coronary angiography. The patient had reported exertional chest pain prior to this hospital presentation and was awaiting an outpatient coronary angiogram.
They had a background history of hypertension and high cholesterol, and a strong family history of premature atherosclerosis, with their sister requiring percutaneous coronary intervention (PCI) at 60 years of age and their father having a coronary artery bypass graft at 52 years of age. The patient was a non-smoker.
Their pre-hospital medications included atorvastatin 40 mg, pantoprazole 40 mg, lercanidipine 10 mg, bisoprolol 2.5 mg, and aspirin 75 mg.
The differentials that were considered at this point included: ACS; Takotsubo cardiomyopathy; myocarditis; pulmonary embolism; and aortic dissection.
INVESTIGATION AND MANAGEMENT
The patient underwent coronary angiography via a 6 Fr right radial approach. This revealed a normal left main stem, a 90% ostial lesion of the left anterior descending coronary artery (LAD), a co-dominant left circumflex artery with moderate atheroma of an obtuse marginal artery branch, and a proximal right coronary artery lesion of approximately 70% (Figure 1).
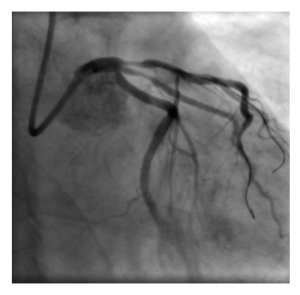
Figure 1: Original left anterior descending artery stenosis.
The authors proceeded to PCI of the LAD. The LAD was wired with a sion wire and a second sion wire was placed in a large diagonal branch.
The authors attempted to direct stent the proximal LAD lesion with a 3.5×24.0 mm Promus stent (Boston Scientific, Galway, Ireland). The stent travelled into position easily and was connected to the inflation system; however, when the authors went to deploy the stent, the balloon of the stent did not inflate. At this point, they attempted to withdraw the entire stent apparatus from the vessel, but the balloon and stent remained in situ. They discovered on further withdrawal and removal from the patient that there was a fracture in the delivery shaft, leaving behind residual shaft, balloon, and undeployed stent in the left main (LM) or LAD (Figure 2).
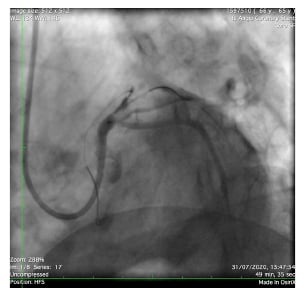
Figure 2: Failed left anterior descending artery stent deployment.
At this point the patient became unstable, likely due to occlusive flow in the LAD from the residual balloon and stent, developing ST elevation with VT and loss of cardiac output. They required emergency cardioversion, were given intracoronary adrenaline, and anaesthetics were called.
The authors attempted to snare the residual shaft in the aortic root from the right radial with an Nsnare™ Stent Retriever (Cook Medical, Bloomington, Indiana, USA); however, they could not capture it despite guide manipulation and wire retraction. Of note, the authors could not visualise the residual shaft, it being radiolucent. The patient developed severe right subclavian spasm following these manipulations, and the Nsnare could no longer advance nor retract.
The authors established right femoral arterial access with an 8 Fr sheath. They again attempted to snare the residual shaft with an Nsnare, followed by an Amplatz Goose Neck™ snare (Medtronic, Watford, Hertfordshire, UK), and an IR snare, using a JL3.5 guide, then switching to a Q3.5 guide to change possible snare angles in the aortic root (Figures 3 and 4).
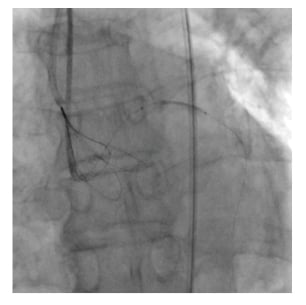
Figure 3: Attempted retrieval with an NsnareTM Stent Retriever (Cook Medical, Bloomington, Indiana, USA).
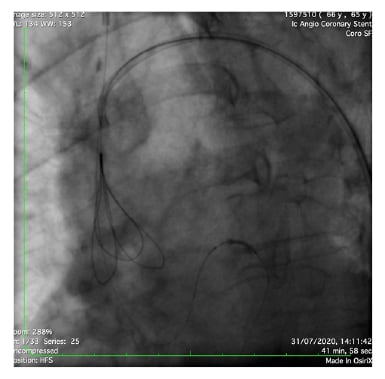
Figure 4: Further attempts at retrieval with an NsnareTM Stent Retriever (Cook Medical, Bloomington, Indiana, USA).
Thereafter, the authors tried another approach, where they advanced a coronary wire past the undeployed stent in the LM artery, that travelled into a diagonal branch. They used serial balloon inflations (1.5×12.0 mm, 2.0×12.0 mm, and 2.5×12.0 mm) over this wire with aggressive traction to try and pull back the undeployed stent into the aortic root. The stent did move slowly backwards.
The LAD was wired with the aid of a turnpike spiral microcatheter. The authors attempted to wire through the stent with a Pilot 200 (Abbott Vascular, Maidenhead, Berkshire, UK), and Confianza® Pro 12 (Asahi Intecc USA, Inc., Santa Ana, California, USA), but the turnpike spiral would not pass through.
The authors removed the wire and turnpike spiral and repositioned the guide. The guide manipulation moved the stent into the aortic root; the stent was then captured by an Nsnare in the aortic root and pulled into the Q3.5 guide.
Left femoral arterial access was obtained with an 8 Fr guide, with iliac crossover to maintain site control in case of access complication.
A Terumo stiff wire (Terumo Interventional Systems, Somerset, New Jersey, USA) was placed in the right femoral, and the guide was retracted gently into the 8 Fr femoral sheath and removed. A femoral angiogram from the left showed no site complications.
The patient was transiently hypotensive and required atropine and metaraminol, likely from a vagal response to pain at the site on sheath removal (Figure 5).

Figure 5: Fractured stent and shaft post-removal.
At this point, the authors returned to the LAD lesion. They passed a sion wire into the LAD. They placed a 3.0×28.0 mm XIENCE drug-eluting stent (Abbott Vascular) to the mid-LAD at 12 ats, and a 3.5 x 28 mm XIENCE drug-eluting stent to the proximal LAD or LM arteries at 16 ats. They assessed with intravascular ultrasound (IVUS) to guidepost dilatation, and post-dilated with a 3.5 mm OPN NC balloon (SIS [Swiss Interventional Systems] Medical, Frauenfeld, Switzerland) in the proximal LAD to 20 ats, and a 5.5×8.0 mm NC balloon in the LM to 20 ats.
There was a non-flow limiting dissection noted in the diagonal branch outflow with thrombolysis in myocardial infarction (TIMI) 3 flow throughout; therefore, the authors did not treat this.
They then removed the original Nsnare from the right radial without issue, as the spasm had resolved. The femoral arterial access sites were closed bilaterally with 8 Fr ANGIO-SEAL® (Terumo Interventional Systems) devices. A TR Band® (Terumo Interventional Systems) was applied to the right radial. A bedside transthoracic echocardiogram showed no pericardial effusion.
The entire procedure took over 6 hours.
Shortly after the end of the procedure, the patient reported a left arm weakness and mild speech slurring. They were emergently reviewed by the on-call stroke team who noted an National Institutes of Health Stroke Scale (NIHSS) score of 3. A CT-brain and CT-cerebral angiogram were arranged, which showed no acute abnormality. An MRI-brain scan did show widespread scattered embolic type infarcts, from which the patient made a complete functional recovery. Their peak troponin following this event was 954, and a transthoracic echocardiogram showed a preserved left ventricular ejection fraction of 55–60%, without regional wall motion abnormalities noted. The patient had a short course of antibiotics for aspiration concerns during the cardiac arrest and had inpatient IVUS-guided PCI to the right coronary artery 11 days after the initial procedure. The LAD or diagonal stents were also reassessed with further IVUS guided post-dilatation. The patient was discharged home well the following day.
DISCUSSION
PCI in the field of cardiology has significantly advanced in recent years, and has been proven to have superior outcomes to medical management or thrombolysis in the setting of ACS.1,2 Despite equipment and technical improvements, complications, through downtrending, remain an ongoing clinical challenge as cardiologists encounter increasing patient comorbidities and lesion complexity.3,4 Stent shaft fracture and retention of coronary interventional products are rare, but serious complications of percutaneous angioplasty can result in emergency surgical procedures, or death.5 It is essential for interventional cardiologists to have a thorough understanding of all equipment being used in the catheterisation laboratory, an awareness of the potential for equipment complication to occur, and knowledge of how to deal with such complications. It is also imperative to continuously assess equipment in use in the catheterisation laboratory during a procedure, to ensure there has been no damage sustained that could impair function, or increase the risk of complications.
The incidence of stent loss has significantly reduced, with the development of second- and third-generation stents recently quoted at 1.3%.6 Stent delivery shaft fracture is very rare, with only case reports noted. Issues contributing to such a complication can include physicians, such as excessive manipulation or pushing; patients, such as difficult delivery, tortuosity, and calcification; and equipment being possibly faulty from manufacture.
Retrieval techniques described in case reports include use the of snare and forceps devices, alongside the use of wire techniques such as the double helix for entrapment, the small balloon technique where a balloon is inflated distal to the stent and withdrawn, and the use of balloon inflation in the guide to compress and withdraw the retained components.7-9 Interventional cardiologists should have basic knowledge of these techniques in the event of such a complication, as different situations are likely to require different techniques.
FOLLOW-UP
The patient has been contacted via telephone and is doing well in the outpatient setting, without residual neurological or cardiac symptoms.
CONCLUSIONS
This case highlights the challenges of managing this rare complication, and reminds us of the need for interventional cardiologists to remain upskilled in the management of unusual complications, as this can prevent the need for patients to proceed to emergency surgery.







