INTRODUCTION
Percutaneous treatment of coronary chronic total occlusions (CTO) has considerably advanced over the last decades, thanks to the development of dedicated technologies and improved skills of experienced operators. Although randomised evidence on hard endpoints is lacking, the key role of CTO recanalisation in relieving symptoms and improving quality of life has been widely demonstrated. Besides, there is still a need for optimising risk assessment and patient selection, and implementing tailored treatment strategies according to lesions and patients’ characteristics. Accordingly, the authors discuss the potential role of invasive coronary physiology assessment and coronary CT angiography in guiding management and decision-making in CTO clinical practice, as well as new strategies to minimise stent implantation in CTO lesions. Percutaneous coronary intervention (PCI) of CTO is one of the most heatedly debated topics in interventional cardiology. Indeed, CTO–PCI raises unique procedural challenges and questions about the best strategy. Considering the recent development in invasive coronary physiology assessment and coronary CT angiography (CCTA) planning for structural interventions, the authors believe these relatively new tools may have their role in guiding management and decision-making in CTO clinical practice. Moreover, given the well-known higher risk of target vessel failure and need for revascularisation in CTO–PCI compared with non-CTO–PCI (partly due to more extensive stenting in CTO–PCI), the authors believe that new solutions are needed to minimise stent implantation in this procedure. The authors deem those as some of the most attractive questions about the future directions for CTO–PCI and, accordingly, they provide their opinion on the topic.
Despite the evolution in technology and techniques, CTO prevalence has remained stable, at around 20% of patients undergoing coronary angiography.1 Moreover, in the presence of an ageing population, and the increased survival of patients with complex coronary artery disease, it is anticipated that a growing proportion of patients will present with CTO lesions. Although the success rate of CTO revascularisation has increased in the last decade (more than 90% in experienced operators’ hands),2 accompanied by a substantial reduction in complication occurrence (less than 3% of cases),3 the indication itself to CTO–PCI remains a matter of debate. According to current literature, CTO–PCI leads to consistent symptomatic relief and quality of life improvement;2 however, the available trials to date were not adequately powered and designed to assess the impact of CTO–PCI on hard endpoints, such as death and myocardial infarction.4-8 Therefore, patient symptoms are currently the main drive for CTO recanalisation, along with signs of inducible ischaemia and myocardial viability in the territory of the CTO,9 that should be confirmed especially in patients with reduced left ventricular ejection fraction.10
Besides, the authors believe that the optimisation of risk assessment and patient selection might further improve the expected clinical benefit, and inform on futility of interventions, thus posing a special need for tailored treatment strategies according to lesions and patients’ characteristics (Figure 1).
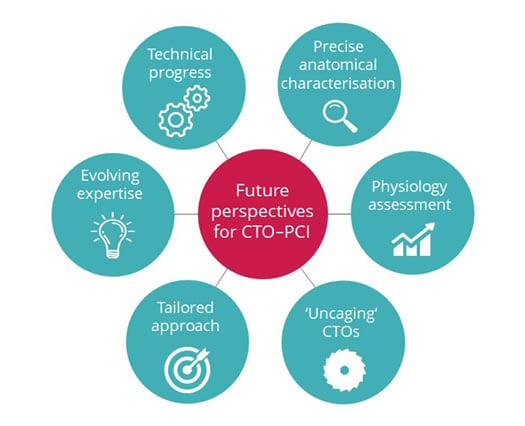
Figure 1: Future perspectives for chronic total occlusion–percutaneous coronary intervention.
Future perspectives for CTO–PCI entail further advancing technologies, evolving expertise of dedicated operators, broadening the adoption of multidimensional and multidisciplinary assessment with integration of physiology, and anatomical characterisation, towards a refinement in patient and lesion selection and development of tailored approaches.
CTO: chronic total occlusion; PCI: percutaneous coronary intervention.
IS THERE ROOM FOR PHYSIOLOGY ASSESSMENT IN CHRONIC TOTAL OCCLUSION?
Based on the limited available studies on invasive physiology assessment in CTO lesions by means of hyperaemic or non-hyperaemic indexes (i.e., fractional flow reserve [FFR] and instantaneous wave-free flow reserve, respectively), significant ischaemia has been observed, regardless of well-developed and well-functioning collateral vessels supplying the vital CTO territories11-13 and regional wall motion abnormalities.14 Overall, the ischaemic burden reduced after successful CTO–PCI and improves over time.13,15 Recent evidence has demonstrated a significant correlation between the magnitude of change in post-PCI FFR and angina relief,16 as well as the association between larger gain in FFR with PCI and improved clinical outcomes.17 Specific data on CTO–PCI, albeit scant, have confirmed that suboptimal post-procedural FFR predicts long-term major adverse cardiovascular events.13 Of note, untreated focal or diffuse epicardial disease besides the CTO segment; suboptimal stenting (derived from malapposition, under-expansion, significant dissection, or plaque protrusion); microvascular dysfunction; and the complex interplay between the distal segment, coronary collaterals, and their donor vessel represent all potential pitfalls to be taken into account.
With regard to the microvascular compartment, the available studies showed inconstant improvement in microvascular function immediately after CTO recanalisation and during follow-up.18,19 Thanks to the recent introduction of reproducible and easy-to-use tool, allowing to accurately quantify the absolute coronary blood flow and microvascular resistance, new findings on distal coronary artery physiology and microvasculature in recanalised CTO vessels are becoming available.20,21 Future research will hopefully expand the knowledge about physiology in CTO, and shed light on the contributing factors to epicardial and microvascular dysfunction and their impact on clinical outcome in this specific setting, thus identifying those CTOs likely or unlikely to benefit from recanalisation, as well as features to guide CTO–PCI procedural and clinical optimisation. In this regard, the ongoing IMPACT-CTO 2 trial22 will provide a substantial contribution.
CORONARY CT ANGIOGRAPHY-GUIDED CHRONIC TOTAL OCCLUSION–PERCUTANEOUS CORONARY INTERVENTION: HOW FAR ARE WE?
CCTA has been increasingly used worldwide to diagnose and rule out coronary artery disease.23 In the context of CTO, CCTA has several potential applications beyond diagnosis, as it can be useful for prediction of procedural success, pre-procedural planning, and intra-procedural guidance (Figure 2).24
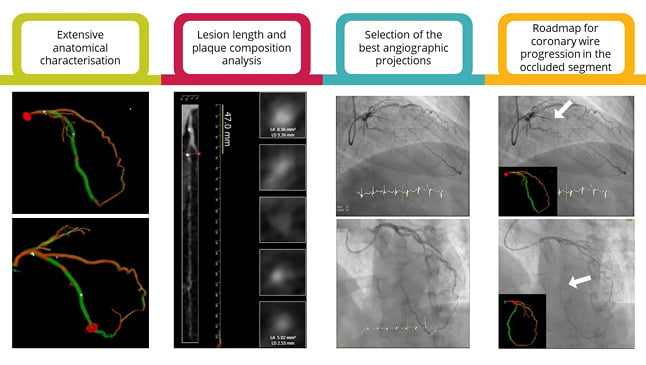
Figure 2: The added value of coronary CT angiography in chronic total occlusion percutaneous revascularisation.
CCTA is a promising technology for enhanced CTO PCI planning and guidance as it allows for the extensive anatomical characterisation (e.g., occlusion length, tortuosity, stump morphology, angulation, calcification burden and cross-sectional area, and outlet and distal vessel morphology); the computation of pre-procedural FFR and prediction of PCI results; the identification of the best angiographic projection in the cath lab, minimising foreshortening and overlapping of the segment of interest; and the identification of the exact vessel trajectory and real-time integration of 3D CCTA data and fluoroscopic images in the cath lab, guiding coronary wire progression in the occluded segment (the white arrows are pointing the coronary wire tip).
Cath lab: catheterisation laboratory; CCTA: coronary CT angiography; CTO: chronic total occlusion; FFR: fractional flow reserve; PCI: percutaneous coronary intervention.
Firstly, CCTA-derived scores resulted to be even more accurate than the traditional angiography-derived J-CTO score (that considers proximal cap shape, calcification, bending >45 °, occlusion length, and previously attempted PCI) for successful CTO–PCI prediction.25-27
Secondly, providing deep insight into the anatomical architecture (e.g., calcification, tortuosity, length, and stump morphology), CCTA might guide best upfront strategy selection for revascularisation and likely improve the outcome.28 Additionally, CCTA analysis can aid in preparing the material and catheterisation laboratory (cath lab), and in identifying the most suitable fluoroscopic projection views, complementing conventional angiography by a 3D model and real-time integration of CCTA data in the cath lab.29
Finally, CCTA offers the chance to integrate the anatomical characterisation with derived functional data by means of a specific software (HeartFlow, Mountain View, California, USA). Based on blood flow simulations using computational fluid dynamics, it is possible to derive FFR from CCTA. The recent development and clinical validation of the FFRCT planner tool further enhances PCI planning potential, as it allows for virtual stenting of coronary stenoses and accurate prediction of post-PCI FFR, independent of the disease pattern, calcification, and image quality.30 Nevertheless, the potential usefulness of CCTA to plan and guide interventional procedures in the CTO remains largely unexplored. The ongoing Precise Procedural and PCI Plan trial31 will provide further evidence on this concept by comparing CT-guided PCI to intravascular ultrasound-guided PCI, also including a subset of CTO lesions.
REDUCING METAL LAYERS IN CHRONIC TOTAL OCCLUSION–PERCUTANEOUS CORONARY INTERVENTION: IS LESS, MORE?
Despite the demonstrated long-term efficacy and safety of contemporary new generation drug-eluting stents,32 total stent length, as well as the number of implanted stents, are procedural factors accounting for long-term risk of stent failure both in non-CTO and CTO–PCI.33 Therefore, the potential for reducing metal deployment represents an attractive perspective to minimise late stent-related events and long-term antithrombotic therapy-driven complications. With this background, the implantation of fully bioresorbable scaffolds and the usage of drug-coated balloons were proposed to ‘uncage’ the coronary vessel wall. Concerning bioresorbable scaffolds, there is paucity of evidence on their use in the specific setting of CTO–PCI, with conflicting results,34-36 thus preventing for drawing definitive conclusions.
In contrast, the use of drug-coated balloons has a number of potential treatment advantages supported by recent clinical data, with initial experiences reported also in the context of CTO–PCI.37 In particular, a hybrid approach, with stenting of the CTO body and delivery of antiproliferative drug through drug-coated balloon inflation in the diseased distal vessel avoiding an additional metal layer, seems a promising strategy.
The ongoing Drug-Coated Balloon Coronary Angioplasty Versus Stenting for Treatment of Disease Adjacent to a Chronic Total Occlusion (Co-CTO) trial38 will provide new clues in this regard, randomising 141 patients with a CTO eligible for PCI to a hybrid versus standard stenting strategy.
CONCLUSION
Thanks to the recent technical progress and the evolving expertise of dedicated operators, the success rate of percutaneous CTO revascularisation is steadily rising, accompanied by a decline in complication occurrence. Nevertheless, several challenges remain to be overcome to optimise procedural results and patient selection. In this regard, the integration of precise anatomical characterisation of the lesion, likely by CCTA, with physiological data, obtained either invasively or non-invasively, could be the key to further improvement. Future research and more robust evidence from randomised clinical trials are required to provide definitive guidance about the management and decision-making in the context of CTO–PCI.