Meeting Summary
The symposium was a practical, case-based discussion in which four panellists each presented a real and challenging case regarding dual antiplatelet therapy (DAPT) strategy. The audience and other panel members were invited to give their thoughts on the difficult decisions that arose during treatment.
Case 1, presented by Dr Chevalier, was a man with multivessel disease, enrolled in the Discovery 1TO3 trial.1 He received staged treatment with three stents; strut coverage was evaluated with optical frequency domain imaging (OFDI). Four days after the staged percutaneous coronary intervention (PCI), the patient suffered a Bleeding Academic Research Consortium (BARC) Type 3a gastric bleeding event and the gastroenterologist requested that the DAPT be stopped.
Dr Vranckx presented Case 2, in which a man, aged 84 years, presented 5 hours after myocardial infarction. He had a bifurcation lesion and was treated with a two-stent technique. Bleeding and ischaemic risk scores varied according to the risk scoring system used, but overall this patient had a high bleeding risk (HBR) and reasonable ischaemic risk. Treatment had to balance the two risks.
Case 3, presented by Dr Cuisset, was a 62-year-old male with chronic coronary artery disease (CAD), alcohol abuse, and Type 2 diabetes mellitus with poor adherence to metformin. The patient had good haemoglobin levels and renal function, and moderate liver abnormalities. He had thrombocytopenia and stable angina with prior documentation of ischaemia under stress. There was no significant lesion on the left anterior descending artery (LAD) or left coronary artery, but a 60–70% stenosis of the mid-right coronary artery (RCA). He was likely to be nonadherent to antiplatelet therapy.
Dr Colombo presented the fourth case, in which a 73-year-old male had a large thoracoabdominal aortic aneurysm and had been referred from vascular surgeons. He had standard risk factors for CAD, reasonable ejection fraction (EF), and no significant valvular disease. Preoperative coronary angiography showed a noncritical stenotic lesion on RCA, a 90% stenotic lesion mid-left circumflex artery (LCX), and total occlusion of LAD. PCI ahead of vascular surgery could involve one, two, or three vessels.
Case 1: Many Stents, An Emergency, and Now What?
Doctor Bernard Chevalier
Case Description
Case 1 was a male with risk factors for dyslipidaemia and hypertension for which he was taking fluvastatin and ramipril, respectively. He had no significant comorbidity. His angina was symptomatic and was Canadian Cardiovascular Society (CCS) Grade III but had been stable for 3 months. Thallium scintigraphy had shown anterior and septal ischaemia and an EF of 58% at rest that reduced to 50% at stress. Diagnostic angiography showed intermediate lesions on the distal left main, proximal, and very distal lesions but nothing notable on the LCX. Of the two regions on the right, one was mid segment and one just before the crux. Fractional flow reserve (FFR) from left main to LCX was 0.85 and it was therefore unnecessary to consider the left main for revascularisation. The syntax score without taking account of the left main lesion was 15. Syntax II scores were 29 for PCI and 30.5 for coronary artery bypass graft.
The patient was enrolled in the DISCOVERY 1TO3 trial1 of PCI and received a loading dose of clopidogrel or aspirin. The post-PCI regimen included clopidogrel for 6 months, aspirin, ramipril, and rosuvastatin. DISCOVERY 1TO3 included 60 patients with multivessel disease. They received staged treatment with the Ultimaster® drug-eluting stent (DES) (Terumo Corporation, Tokyo, Japan). Strut coverage was evaluated with OFDI at baseline when the first stent was implanted, at 1 month at staged stenting, and at 3 months. This gave 1 and 3-month data on the first stent and 2-month data on the staged stent.1
The patient in this case had PCI of the LAD at baseline with Ultimaster 3.0×24 mm with high pressure post dilatation because of calcification. A month later the two lesions on the right were addressed with Ultimaster® 3.0×18 mm and 3.5×15 mm, both pre and post-dilatation. Four days after the staged PCI, 36 days after the LAD PCI, the patient had significant haematemesis with a 4.5 g/dL drop in haemoglobin, defined as a BARC 3a bleeding event. He was haemodynamically stable, transfused with two packs of blood, and received intravenous proton pump inhibitor (PPI). Emergent gastroscopy revealed a gastric ulcer with arterial bleeding, which was treated with electrocoagulation. Two days later, a control gastroscopy showed persistent mild bleeding and the gastroenterologist requested DAPT cessation.
Discussion Point 1: Dual Antiplatelet Therapy Strategy Following a Gastrointestinal Bleed
The team’s options were to stop one antiplatelet agent, either aspirin or clopidogrel, or both; the decision could be based on platelet testing or the OFDI. Alternatively, the gastroenterologist’s request could be refused and DAPT continued.
The question was posed to the audience, and just under half said they would stop aspirin. Nobody would stop clopidogrel or both drugs. On the panel, Dr Cuisset said he would stop aspirin, noting that the gastric ulcer had been treated and the bleeding was mild and did not justify stopping DAPT altogether 4 days after PCI. Dr Vranckx commented that he would continue both aspirin and clopidogrel, along with the PPI, and keep the patient under high surveillance. Dr Chevalier said OFDI performed 1 month after the LAD PCI had shown relatively good strut coverage of 83.1%. The first baseline OFDI on the RCA had shown a proximal malposition in the distal lesion and a distal malposition in the proximal stent. A 4 mm balloon was inserted, and a second evaluation showed it had corrected the malapposition.
The medical team decided to stop the aspirin and start high-dose PPI with gastroscopic control and biopsy at 4 weeks. Clopidogrel alone was maintained for 3 months, as outlined in the study design. Evaluation at 3 months showed good coverage in both LAD and RCA. This is in line with overall results from the DISCOVERY 1TO3 trial (Figure 1), which has shown good strut coverage rates: 85.8±11.7% at 1 month, 89.0±9.9% at 2 months, and 95.7±4.4% at 3 months (p<0.001).1
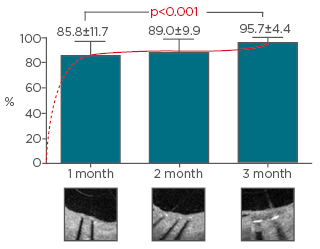
Figure 1: Optical frequency domain imaging was used to assess the percentage of covered struts 1, 2, and 3 months after implantation.
Adapted from Chevalier et al.1
Discussion Point 2: The Long-Term Prescription
The options were ramipril and rosuvastatin, clopidogrel, and/or PPI.
Dr Chevalier said he routinely checks sensitivity to clopidogrel with the platelet function analyser (PFA-100) system when aspirin is stopped. However, Dr Smits noted that the test is not widely available. Dr Colombo agreed, adding that testing is mainly used in special circumstances; he said he would continue clopidogrel in HBR patients because bleeding is prothrombotic and a second bleeding episode could cause thrombosis. Dr Valgimigli noted, however, that current guidelines give only a Class 2B–C recommendation for using the test in cases like the one under discussion, and a Class 3 recommendation for routine platelet function testing; he would continue with clopidogrel monotherapy. Dr Cuisset said response to clopidogrel has a clear impact on clinical outcome in the early post-PCI period, but for stable patients the impact of the test on clinical outcome is weaker, suggesting it may not be useful in secondary prevention. Dr Chevalier clarified that PPI was added at the time of the bleeding event rather than at DAPT initiation, as the case study was before the introduction of new guidelines.2
Conclusions and Take-Home Message
Dr Smits concluded that the OFDI showed that most the of strut coverage took place in the first month after implantation of the Ultimaster stent in the DISCOVERY 1TO3 study.1 Dr Chevalier noted that OFDI may help correct a hidden malapposition and that, even in non-HBR patients, the choice of a DES with quick strut coverage may help deal with a difficult situation. He also reminded the audience not to forget PPI as a preventive action.
Case 2: Bleeding or Clotting, Calculate the Risk
Doctor Pascal Vranckx
Case Description
Case 2 was an 84-year-old male who presented at the emergency department around 5 hours after MI, according to the ECG. He had a history of hypertension treated with an angiotensin-converting enzyme inhibitor in combination with a calcium antagonist, hypercholesterolaemia treated with a statin, and non-insulin-dependent diabetes mellitus treated with metformin. He was a former smoker and had chronic renal failure with a reduced estimated glomerular filtration rate of 42 mL/kg/1.73m2. He had a haemoglobin level of 10.8 g/dL and white blood cell count of 7.2×109/L.
The patient had a bifurcation lesion (MEDINA 1.1.1) and was treated with a two-stent PCI.3 Bleeding risk was weighed against thrombotic risk. In terms of the bleeding risk, risk factors included pre-existing anaemia, previous bleeding, age, and renal function. For the thrombotic risk, risk factors included age, renal function, and clinical presentation, ST-elevation MI (STEMI) versus non-STEMI, blood pressure, and heart rate.
There are different bleeding and thrombosis risk evaluation scores. In clinical practice, bleeding scores were used more often to evaluate bleeding risk, while thrombotic risk was rarely used. Only five members of the audience reported using a thrombotic risk score in routine clinical practice, more reported using a bleeding risk score, and only one member of the audience reported using both risk scores.
Dr Vranckx noted that bleeding translates into mortality, as demonstrated in a post-hoc analysis in the Tracer trial,4 which mapped the cumulative mortality over the months since the bleeding for different BARC and Global Utilization of Streptokinase and Tpa for Occluded Arteries (GUSTO) bleeding criteria. All criteria translate into mortality risk, but the score determines the level of risk: mortality risk associated with GUSTO severe is 30% at 2 years, compared with <10% for a BARC2 bleed (Figure 2).
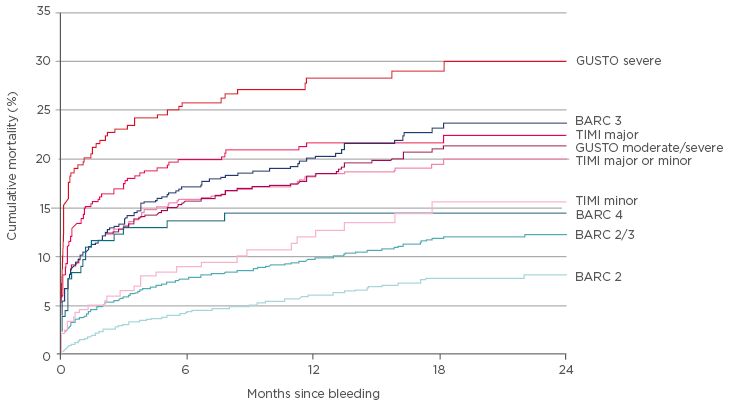
Figure 2: Mortality risk rises as the score on the BARC criteria increases.
BARC: Bleeding Academic Research Consortium; GUSTO: Global Utilization of Streptokinase and Tpa for Occluded Arteries; TIMI: thrombolysis in myocardial infarction.
Adapted from Vranckx et al.4
A major difference between the PRECISE-DAPT (Predicting bleeding complications in patients undergoing stent implantation and subsequent DAPT) score and the DAPT score, recommended by the European Society of Cardiology (ESC) guidelines,2 is that PRECISE-DAPT allows calculation of risk at the time of index procedure, whereas the DAPT score is used after 12 months of uneventful treatment to calculate the risks of continuing with DAPT. The patient in this case study had high PRECISE-DAPT score.5
Discussion Point: Dual Antiplatelet Therapy Strategy Following Myocardial Infarction
The options were to treat for <6 months, 6 months, or 12 months, with a regimen including clopidogrel or ticagrelor.
Nobody in the audience said they would consider DAPT for 12 months with clopidogrel or with the more potent ticagrelor. The majority voted for DAPT for 6 months with either clopidogrel or ticagrelor. Two audience members would consider DAPT for <6 months. Dr Vranckx mentioned that this is the question being addressed by the MASTER DAPT trial.6 Patients in MASTER DAPT received DAPT for 1 month and were then randomised to receive either single antiplatelet therapy (SAPT) for 11 months or DAPT for ≥5 months.
On the panel, Dr Cuisset said he would aim for optimal treatment for the early period because up to 80% of stent thrombosis cases occur within the first month of PCI. He would give aspirin and ticagrelor the first month and switch to the relatively weak P2Y12 inhibitor clopidogrel, with aspirin, for up to 6 months.
Dr Valgimigli said that he would not de-escalate the potency of the P2Y12 inhibitor but, rather, reduce the duration of DAPT. He would give aspirin and ticagrelor or aspirin and prasugrel for 1–3 months, followed by P2Y12 inhibitor monotherapy. Since initial presentation was a STEMI, if the patient was stable, he would give clopidogrel rather than ticagrelor or prasugrel.
Dr Colombo commented that he would have given clopidogrel for at least 6 months because he would be more concerned about the bleeding risk and, without HBR, he might have continued it for 12 months. Using two stents in a bifurcation lesion is a good reason to use DAPT, and ticagrelor is a reasonable alternative to clopidogrel, he said. Because the stenting results are good, and current-generation stents have thin struts, the risk of thrombosis is <1% and clopidogrel should be sufficient; therefore, why subject the patient to the risk of bleeding? Dr Chevalier said this case is a good example of drug–device interaction. He would use clopidogrel if provisional stenting was successful; otherwise, two stents plus ticagrelor.
The team’s choice was driven by the balance between bleeding and ischaemic risk and the patient was randomised for treatment in the MASTER DAPT study.6 This is a randomised trial to compare, within current guidelines and instructions for use, an abbreviated versus a prolonged DAPT duration after bioresorbable polymer coated Ultimaster sirolimus-eluting stent implantation in patients presenting HBR features. The team decided that, if the patient was in the standard DAPT arm, they would be treated for 6 months with ticagrelor preferred to clopidogrel because of the ischaemic risk. At 365 days post-randomisation, the patient had no bleeding or thrombotic problems.
Conclusions and Take-Home Message
Dr Vranckx concluded that DAPT reduces stent thrombosis and ischaemic events after PCI but increases the risk of bleeding. Since the risk factors for ischaemia and bleeding largely overlap (±20%), the duration of DAPT post-stenting should be driven by the balance between the two. The PRECISE-DAPT score, a standardised five-item risk score calculated at the time of the index PCI can provide a standardised tool for predicting out-of-hospital bleeding during DAPT.
Case 3: Should We Go Beyond Necessary?
Doctor Thomas Cuisset
Case description
Case 3 was a 62-year-old male with new-onset angina, CCS Grade II–III. The patient had a history of Type 2 diabetes mellitus, active smoking, and chronic alcohol abuse. He was taking metformin for the diabetes, but no antiplatelet therapy. According to the family, his adherence to metformin was irregular. Echocardiography showed good left ventricular EF (LVEF) function (65%) with no significant valvular disease; however, stress testing showed abnormalities in the inferior wall with significant ST changes >1 mm. Laboratory results showed good haemoglobin levels (12.7 g/dL), but platelet count was 120×109/L. There was good renal function, moderate liver abnormalities, and no coagulation disorder. The cathlab found no significant lesion on either LAD or left coronary artery. There was also 60–70% stenosis of the RCA.
Discussion Point 1: Options for Revascularisation
The options were to offer optimal medical treatment rather than PCI; to first confirm the stress echo with invasive or non-invasive functional assessment instantaneous wave-free ratio and FFR; or to offer PCI of the RCA.
Just over half of the audience would carry out PCI, but a substantial minority would opt for optimal medical treatment; the risk of bleeding and of nonadherence to DAPT in this patient would support that approach. However, Dr Cuisset’s team decided, because of the symptoms and documented ischaemia, to proceed with PCI of the RCA. HBR patients can be treated with new-generation DES, which allows for short or very short DAPT duration. He said FFR should not be over-used and was not necessary in a patient with single vessel disease and symptoms.
Discussion Point 2: Ad-hoc Versus Staged PCI and Antiplatelet Strategy
Having decided to offer PCI of the RCA, the options were:
- Ad-hoc PCI with a loading dose of clopidogrel (600 mg) just after PCI. Discharge DAPT with aspirin plus clopidogrel.
- Ad-hoc PCI with a loading dose of ticagrelor (180 mg) post PCI. Discharge DAPT with aspirin plus ticagrelor.
- Staged PCI and take time to discuss with the patient and family. A loading dose of clopidogrel (600 mg) the day before PCI, and discharge DAPT with aspirin plus clopidogrel.
- Staged PCI with more potent P2Y12 inhibitor (e.g., ticagrelor 180 mg loading dose) the day before PCI, and discharge DAPT with aspirin plus ticagrelor.
There was no clear preference among the audience, but Option 1 attracted the most votes. Panellist Dr Vranckx chose Option 4 because the patient is an alcoholic with liver disease. Since clopidogrel is metabolised by the liver, he would choose a P2Y12 inhibitor that bypasses it. Dr Valgimigli said this strategy is innovative, but, because the patient is relatively HBR with liver cirrhosis and thrombocytopenia above the cut-off point for bleeding, he would avoid ticagrelor or prasugrel and would give aspirin and clopidogrel instead. Dr Smits commented that since the lesion looked stable, he would also go for clopidogrel.
Option 1 was chosen, and the procedure was carried out ad-hoc without invasive functional imaging. The Ultimaster DES 2.75×15 mm was implanted with good angiographic result. Ticagrelor was not chosen because of the HBR and because there is little evidence for the new P2Y12 inhibitors in elective PCI in stable patients. Instead, a loading dose of 600 mg clopidogrel was given post PCI and the patient was discharged with aspirin plus clopidogrel.
Discussion Point 3: Dual Antiplatelet Therapy Duration at Discharge When Nonadherence is Likely
The options were a priori DAPT duration for 1 month, 3 months, 6 months, or longer. Most of the audience favoured 3 months for this patient at discharge; however, some opted for 1 month, others for 6 months.
On the panel, Dr Colombo said he would choose 3–6 months’ DAPT, but his experience suggested the patient would stop all treatment after a week. He would use any new-generation stent, possibly a polymer-free stent or even bare-metal stent (BMS) for this short (<3 mm), single lesion with a low risk of restenosis of 15–20%. Dr Chevalier disagreed. He would choose a stent with quick coverage, such as Ultimaster DES, and 3 months DAPT, aiming to see the patient at 1 month to convince him to continue treatment. He said there is no role for BMS in any type of lesion now, even though they were used in the past. Dr Valgimigli said the evidence to support this point of view exists and the only study suggesting otherwise is NORSTENT.7 His group is conducting a meta-analysis of data from >30,000 patients, including those in NORSTENT, randomly allocated to thin-strut BMS versus new-generation DES. The results are not yet available, but suggest that BMS should not be used.
Dr Cuisset agreed with the audience’s decision to opt a priori for 3 months’ DAPT. The MASTER DAPT study is ongoing but there is not yet evidence for a 1-month default strategy. The HBR patient of this case study was not at the same bleeding risk as a patient on anticoagulation, a very elderly patient, or someone with prior or active bleeding. Additionally, 6 months of DAPT was too long because of the clear risk of both bleeding and nonadherence.
Furthermore, the patient had mild thrombocytopenia (100–150×109/L), and a paper by McCarthy et al.8 indicated that bleeding prevention in HBR patients, such as the one under discussion, should be done with PPI. Glycoprotein IIb and IIIa inhibitors should be avoided, low-dose aspirin should be given, and DAPT duration after new-generation DES should be shortened to 1–3 months. Also, a transradial approach with a new generation DES should be used. In light of this, Dr Cuisset’s group used Ultimaster with 3 months’ DAPT, as the strategy decided at discharge.
The patient and his family were educated on adherence and the patient’s general practitioner was involved. A fixed-dose combination of aspirin and clopidogrel reduced the number of pills taken. Unfortunately, 6 weeks after PCI, the patient arrived in the emergency department, drunk, with trauma, minor bleeding, and a BARC2 bleeding complication. He described a skin haematoma since hospital discharge, which indicated that he had adhered to the DAPT.
Discussion Point 4: Dual Antiplatelet Therapy Strategy After a BARC2 Bleed
Options at this stage were to continue with the original 3-month DAPT strategy, stop aspirin and/or clopidogrel, or both. Alternatively, platelet testing could be used to aid the decision.
Some of the audience said they would continue with DAPT, and a similar number would stop aspirin alone; a few would stop the clopidogrel, and no one would stop both. A handful would like to use platelet function testing to test adherence.
Dr Cuisset said the patient’s HBR, coupled with BARC1 and 2 level bleeding, which would further increase risk of nonadherence, led them not to continue their original strategy. Stopping either aspirin or clopidogrel without platelet function test could be a good compromise. Stopping both would leave the patient at risk of stenosis. The team therefore stopped clopidogrel, continuing aspirin as SAPT.
Conclusions and Take-Home Message
New-generation DES with short duration DAPT allowed for easier management of a challenging patient and many others with HBR. DAPT duration should be tailored according to the individual patient, the stent used, and the complexity of the PCI. The DAPT strategy chosen immediately post PCI is a dynamic decision which should be adjusted according to the outcomes including the patient’s drug tolerance.
Case 4: When the Number of Stents and Months of Dual Antiplatelet Therapy Should Not Match
Doctor Antonio Colombo
Introduction
Dr Colombo first stated that the long-term success of PCI depends on optimum antiplatelet therapy, a well-implanted stent, and good distal flow. The three variables need to be optimised; it is not recommended to compensate for a weakness in one by increasing the strength of another. In 1998, the first registry study to look at BMS implantation with single agent aspirin, found only two stent thromboses in the first week after stenting.9 Aspirin alone would not now be considered in PCI, but the study shows the value of optimal implantation technique. More recently, meta-analyses of intravascular ultrasound (IVUS) versus angiography have shown that stent thrombosis is significantly reduced where IVUS is used rather than angiography.10,11 Even so, IVUS is not used routinely.
Case Description
Case 4 was a 73-year-old male with a large thoracoabdominal aortic aneurysm (57 mm diameter), who had been referred by vascular surgeons. He had no prior history of bleeding, standard risk factors for CAD (hypertension, dyslipidaemia, ex-smoker), nothing remarkable in laboratory data (haemoglobin: 14.7 g/dL; creatinine: 1.13 mg/dL; estimated glomerular filtration rate: 49.1 mL/min/1.73m2), reasonable EF (54%), and no significant valvular disease. The planned surgical operation was replacement of the aneurysm with a synthetic graft within a few weeks or months.
Angiography showed a stenotic lesion on the RCA (75%) that was not critical and could have been negative on FFR. There was a significant stenotic lesion (90%) on the mid LCX and total occlusion of the LAD.
Discussion Point 1: The Role of Preoperative Coronary Angiography
Preoperative coronary angiography was carried out in this case, which led to complex decision-making. One member of the audience said there is no evidence for patients undergoing non-cardiac surgery to have routine coronary angiography in the absence of symptoms; only patients with prognostic ischaemia on functional imaging and unstable angina symptoms should have angiography. Patients with aortic aneurysm are routinely treated with endovascular stenting under a spinal or even local anaesthetic, which precludes the need for general anaesthetic and extensive surgery. Once angiography has established that the patient has severe CAD, the situation is difficult; stenting only the straightforward lesions may not eliminate the patient’s risk from general anaesthetic.
Co-chair Dr Valgimigli was not in favour of preoperative coronary angiography, but acknowledged that, in his institution, surgery would not go ahead without it. Dr Colombo added that the evidence on angiograms is lacking. He was not uneasy about preoperative angiograms because of the risk of myocardial infarction in patients operated on by vascular surgeons. Dr Cuisset commented that he would consider the angiogram only if a noninvasive test was positive.
Discussion Point 2: To Intervene or Not to Intervene
Following the angiography, the team’s options ahead of the aortic aneurysm surgery planned in the following weeks or months were to not intervene, to treat the LAD and/or the LCX, and/or the RCA. Most of the audience would intervene, and most would treat the LCX. Dr Cuisset said, once he knew there was triple vessel disease, he would have minimised stenting and called the patient back after vascular surgery to do the second lesion. Dr Chevalier and Dr Colombo both said they would have stented the LCX and nothing else, but this is not what happened. It was decided to opt for LAD CTO recanalisation. After successful wire crossing, ASAHI Gaia Second (ASAHI INTECC CO., LTD, Huddersfield, UK) and predilatation (1.25 mm and 2.5 mm), three stents were put on LAD, and the two-stent technique, guided by IVUS, was used on the LCX bifurcation. No FFR was carried out on the right coronary, but two stents were placed on the RCA. The operator was skilled and it was an excellent result; however, this patient had seven stents before undergoing planned surgery.
Discussion Point 3: Dual Antiplatelet Therapy Strategy Ahead of Vascular Surgery
There was little debate that DAPT was needed after such extensive stenting. The team’s options were to give DAPT for 3 months, 6 months, or 1 year. Some in the audience opted for 3 months, others for 6 months.
Dr Colombo noted that surgery with three vessels open is not a bad idea if the stents are well endothelialised after 3, 4, or 5 months. DAPT was necessary but Dr Colombo said ticagrelor was not needed in this stable patient.
After the staged PCI, aspirin 100 mg and clopidogrel 75 mg were given for 1 month only and then stopped by the vascular team. Cardiac surgery for the thoracoabdominal aortic aneurysm was performed successfully with the patient off DAPT, and afterwards only clopidogrel was restarted. Dr Colombo disagreed with the approach and said that stopping both aspirin and clopidogrel after 1 month is risky. However, 1 week after the operation the patient was discharged without in-hospital thrombotic or bleeding complications.
Conclusions and Take-Home Message
For a patient with HBR, shorter duration of DAPT after PCI is appropriate. New-generation DES with more favourable properties for early endothelialisation including thinner struts, higher biocompatibility, or bioresorbable polymers, may allow shorter duration DAPT more safely. Optimal implantation techniques must be used to achieve the best possible results even with the most reliable device.
Conclusion
Complex decisions on DAPT strategy must be taken in practice. Patients need to be protected from bleeding and ischaemic risks; these risks guide decision-making on DAPT strategy. Risks can be predictable or non-predictable, but even where a high risk of bleeding or ischaemic recurrence is predicted, the treatment paradigm is only changed where there is clear evidence that, by doing so, the risk would be modified. Currently there is insufficient evidence to guide DAPT treatment in challenging scenarios such as in HBR patients. New-generation DES with fast strut coverage might allow short DAPT duration after PCI. An ongoing randomised trial (MASTER DAPT) in HBR patients comparing an abbreviated versus a prolonged DAPT duration after implantation of a new-generation DES, will provide further evidence to help address this challenging clinical question.6








