BACKGROUND AND AIMS
Multidrug-resistant (MDR) bacteria, or ‘superbugs’, pose an impending threat for human health around the world. Common infections are becoming more difficult to treat, with dire consequences for people needing invasive and life-saving surgery. Models predict 10 million annual deaths by 2050 as a result from infectious diseases caused by MDR bacteria.1 Moreover, the majority of bacterial infections are associated with biofilms that, compared to planktonic forms, confer additional 10–1,000-fold tolerance to antimicrobial therapy.2,3 Therefore, new therapeutic strategies are urgently needed to bypass MDR traits of bacteria.
The authors hypothesised that cold-plasma technology has the prospect as a non-pharmaceutical antibacterial and anti-biofilm therapy against MDR bacteria. Plasma discharged in water (plasma-activated water; PAW) forms reactive oxygen and nitrogen species,4 which increase the conductivity and redox potential, and decreases the pH to create an antimicrobial environment capable of effectively killing bacteria. The study herein shows preliminary data on the biofilm-killing capacity of PAW and non-cytotoxicity in human cell culture.
MATERIALS AND METHODS
Five types of PAW (Plasmatreat GmbH, Steinhagen, Germany) were validated for antibacterial and anti-biofilm activity against the ‘ESKAPE’ pathogens Escherichia coli, methicillin-resistant Staphylococcus aureus (MRSA), Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterococcus faecium. The minimum inhibitory concentration of each PAW was determined following standard protocol (broth microdilution). Biofilms were formed over 48 hours before exposure to PAW for 30 minutes. Then, the anti-biofilm activity of PAW was measured by the AlamarBlue viability assay (Invitrogen, Thermo Fisher Scientific, Waltham, Massachusetts) and visualised by confocal microscopy with LIVE/DEAD BacLight staining (Invitrogen, Thermo Fisher Scientific). The viability of human keratinocytes after 30 minutes of PAW exposure was determined with the lactate dehydrogenase viability assay.
RESULTS
Only PAW 1 and 2 effectively inhibited growth of all ESKAPE pathogens, with minimum inhibitory concentration values of 25% and 50% dilution, respectively. Substantial anti-biofilm activity was observed for PAW 1, showing 98% biofilm killing of MRSA (Figure 1A) and 99% biofilm killing of A. baumannii (Figure 1B), while PAW 2 was only effective against A. baumannii (90% biofilm killing). Results in MRSA biofilms were further confirmed by confocal microscopy. PAW 1 treatment substantially reduced the amount of MRSA biofilms and effectively killed bacteria, with the red cells indicating cell death (Figure 1C). Other PAW types were ineffective against planktonic and biofilm bacteria. All PAW types showed no toxicity in keratinocytes.
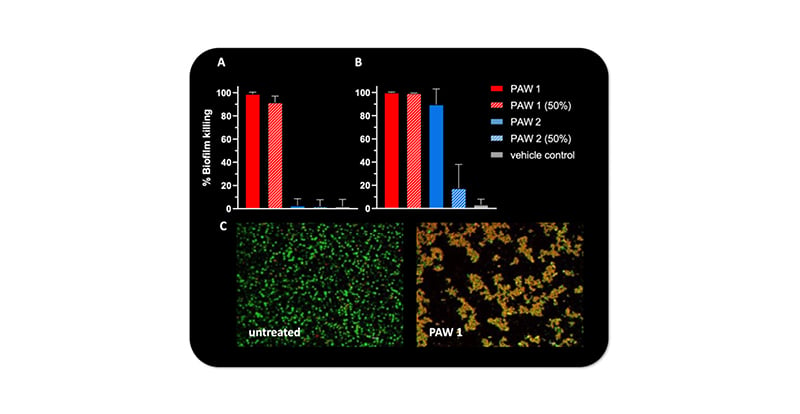
Figure 1: Biofilm killing of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii after plasma-activated water treatment.
Substantial biofilm killing of A) MRSA and B) A. baumannii after exposure to undiluted PAW and 50% diluted PAW in growth media. C) Biofilm killing of MRSA biofilms by PAW 1 compared to untreated control was visualised by confocal microscopy and LIVE/DEAD staining.*
*Green: viable bacteria; red: dead bacteria.
MRSA: methicillin-resistant Staphylococcus aureus; PAW: plasma-activated water.
CONCLUSION
PAW exhibited high efficacy against MDR bacteria and biofilms of ESKAPE pathogens and no cytotoxicity in keratinocytes. These properties present specific types of PAW as a potential new treatment of microbial infections on human tissue, for example as an antibiotic-free lavage for surgical procedures or wounds.








