Meeting Summary
Although treatment for HIV has advanced considerably over the decades, there are still people with HIV that is resistant to multiple antiretroviral therapies (ART). Ibalizumab, a humanized IgG4 monoclonal antibody that gained orphan status in 2018, is a post-attachment inhibitor whose efficacy for people with multidrug-resistant (MDR) HIV has been demonstrated in Phase II and III trials. The Prospective and Retrospective Observational Study of Multidrug-Resistant Patient Outcomes with and without Ibalizumab in a Real-World Setting: United States (PROMISE-US) is an ongoing study that will investigate long-term efficacy and durability of ibalizumab plus an optimized background regimen (OBR). In the poster discussed here, presented at IDWeek 2024, examination of baseline demographics showed that individuals prescribed ibalizumab (IBA group) had similar mean age, sex, race, and duration since diagnosis of HIV to those on regimens that did not include ibalizumab (non-IBA group). However, mean baseline HIV RNA copies/mL were much higher and CD4+ T cells/mm3 lower in the IBA group compared with the non-IBA group, and highest/lowest at baseline in a subset of IBA group patients who were also prescribed the capsid inhibitor lenacapavir. OBR also differed between groups. These results highlight the HIV-related characteristics of individuals who are more likely to be prescribed ibalizumab.
Introduction
In the USA, around 1.1 million people are currently diagnosed with HIV.1 While treatment has advanced greatly,1 a recent analysis including nearly 24,000 individuals found that 9.1% had limited and/or exhausted treatment options.2 Typically, this cohort has multiple ART class exposure, which may be due to HIV with drug resistance,2,3 decreased sequential regimen potency,2,4 adverse effects,2,5 and/or suboptimal treatment adherence.6 As treatment choice becomes limited, maintaining virologic control can be challenging and may lead to grave clinical outcomes.1
The humanized IgG4 monoclonal antibody ibalizumab binds CD4 extracellular domain 2, preventing CD4-HIV envelope glycoprotein 120-induced conformational changes and inhibiting viral entry.7 Ibalizumab lacks cross-resistance with other ARTs, including entry inhibitors.8 Ibalizumab’s efficacy in combination with an OBR has been demonstrated in Phase II and III trials where administration led to large decreases in viral load, including in people with MDR HIV.7,9,10
PROMISE-US Real World Study of Ibalizumab
While ibalizumab gained orphan drug status in 2018,11 clinical trial study numbers were relatively low and administration time was relatively short (up to 48 weeks).4,7,9,10 PROMISE-US is an ongoing, Phase IV, multicenter, retrospective and prospective, observational, non-interventional, registry study in adults with MDR HIV.12 This 3-year, real-world study will investigate long-term efficacy and durability of ibalizumab plus an OBR (IBA group), compared to matched patients not receiving IBA (non-IBA group). The first patient visit was in May 2022, with retrospective data collected from May 2018. Data presented in this poster at IDWeek 2024, collected up to November 8th 2023, provided an insight into the HIV-related status of adults with MDR HIV.
At baseline (latest treatment change event), non-IBA (n=70) and IBA (n=42) group participants had similar mean (standard deviation [SD]) ages of 58.5 (9.71) and 53.8 (10.89) years. Participants were predominantly assigned male at birth (71.4% and 81.0%, respectively), were White (48.6% and 47.6%, respectively) or Black or African American (48.6% and 45.2%, respectively), and were non-Hispanic (84.3% and 78.6%, respectively).
Notable was that mean (SD) duration since HIV+ diagnosis was long and was similar between groups: 26.4 (8.87) years for non-IBA group and 27.7 (8.73) years for IBA group. While respective medians were also similar (28.3 and 30.9 years, respectively), examination of interquartile ranges (IQR; 4.68, 42.42 years and 5.29, 38.84 years, respectively) revealed that some individuals had only been diagnosed for relatively few years while others had lived with HIV for several decades.
As can be seen in Figure 1, at enrollment, mean HIV RNA copies/mL were lowest in the non-IBA group, though still indicative of some virologic failure (>200 copies/mL).13 Viral load was highest in a subset of 12 IBA group participants who, due to more limited ART choices, additionally received the first-in-class capsid inhibitor lenacapavir (IBA/LEN group), a parenteral agent recently approved for people with MDR HIV.14 Of note, SDs in all groups were high, and along with medians (IQR) of 19 (0, 148,000) copies/mL in the non-IBA group, 44 (0, 1,200,000) copies/mL in the IBA group, and 108 (19, 1,200,000) copies/mL in the IBA/LEN group, highlighted between and within group heterogeneity.
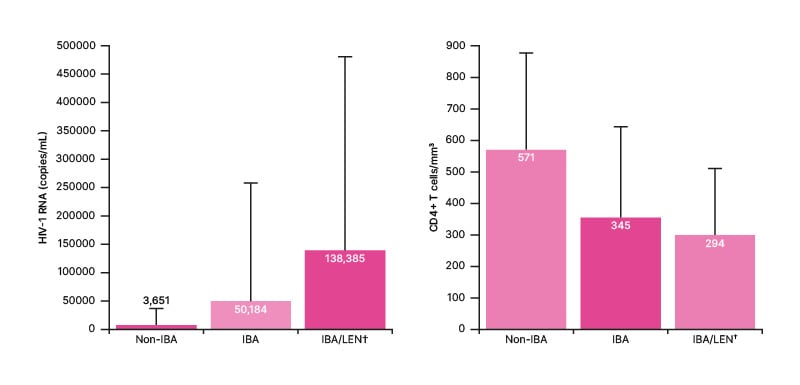
Figure 1: Mean (standard deviation) HIV-1 RNA copies/mL and CD4+ T cells/mm3 at enrolment.
Subgroup of IBA cohort. IBA: ibalizumab; LEN: lenacapavir.
Figure 1 shows how mean and median CD4+ T cells/mm3 were highest in the non-IBA group (571 and 579 cells/mm3, respectively) and were within the healthy range of 500−1500 cells/mm3,15 However, both SD (302 cells/mm3) and IQRs (34, 1,436 cells/mm3) indicated some individuals not receiving ibalizumab were below this range. Respective mean CD4+ T cells/mm3 (Figure 1; SD: 279 and 211 cells/mm3, respectively) and medians [IQR] (283 [13, 1,231] cells/mm3 and 311 [32, 609] cells/mm3, respectively) were similar in the IBA and IBA/LEN groups and indicated that many participants had low counts, with some into the immunocompromised range (<200 cells/mm3).15
The OBR at enrollment for each group is reported in Table 1. For both non-IBA and IBA groups, this primarily included an integrase strand transfer inhibitor, a non-nucleotide reverse transcriptase inhibitor, and/or a protease inhibitor. Two participants in the non-IBA group also received lenacapavir.
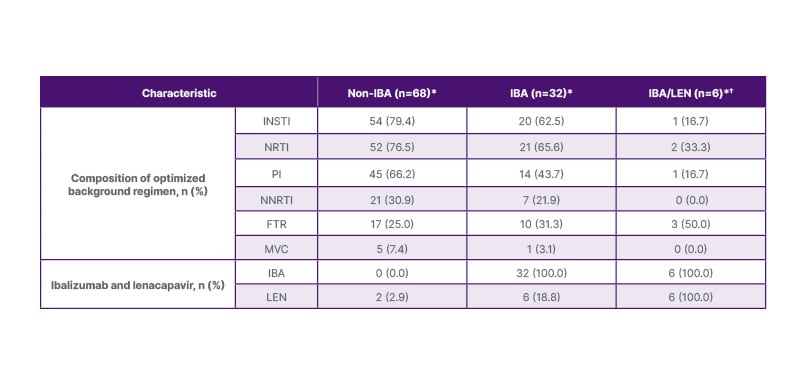
Table 1: Optimized background regimen by group.
*Due to missing data, optimized background regimen is only reported for the subset of participants with complete data entry.
†Subgroup of IBA cohort.
FTR: fostemsavir; IBA: ibalizumab; INSTI: integrase strand transfer inhibitor; LEN: lenacapavir; MVC: maraviroc; NNRTI: non-nucleotide reverse transcriptase inhibitor; NRTI: nucleotide reverse transcriptase inhibitor; PI: protease inhibitor.
Mean [SD] combined genotypic sensitivity score, a measure of ART resistance,16 was lowest in the non-IBA group (2.2 [0.75]), compared with the IBA (2.7 [0.96]) and IBA/LEN (2.9 [1.4]) groups. Commercial resistance testing is not available for ibalizumab, fostemsavir, or lenacapavir, so they are considered fully active and were not included in this score. As such, the authors postulated that “this measure may overestimate regimen sensitivity in IBA group participants who were more likely to have these in their regimens.”
At the time of data collection, around 80% in the IBA group had been taking ibalizumab for >12 months, and around 60% for >24 months. Ibalizumab was well-tolerated with no infusion reactions.
In conclusion, even though IBA group participants had higher viral loads and lower CD4 cell counts at baseline than the non-IBA group, ibalizumab (with or without lenacapavir) demonstrated good durability, with many remaining on this therapy for ≥2 years. PROMISE-US will add to clinical trial results, help further characterize the efficacy and safety profile of ibalizumab plus OBR, and help guide treatment selection for a population with a high unmet need to maintain virological control.







