Meeting Summary
While modern antiretroviral therapy (ART) for people with HIV (PWH) increases life expectancy, there is still an increased risk of developing cardiovascular disease (CVD) in this population. As one factor associated with this increased risk is excess visceral abdominal fat (EVAF), the Visceral Adiposity Measurement and Observations Study (VAMOS) aimed to assess the impact of EVAF on CVD risk in PWH taking modern ART. Participants were grouped according to visceral adipose tissue (VAT) surface area <130 cm2 (non-EVAF group) or ≥130 cm2 (EVAF group), quantified by CT scan. Findings presented at IDWeek 2024 revealed significant differences between EVAF and non-EVAF groups in 10-year atherosclerotic CVD (ASCVD) risk score, as well as many of their individual components. VAMOS also showed correlations between increasing VAT surface area and increasing 10-year ASCVD risk score and insulin resistance measures. Accordingly, VAT may represent a targetable factor to reduce ASCVD risk. Also shown was an inverse relationship between growth hormone (GH) levels and VAT surface area. As GH reductions related to obesity are associated with elevated CVD risk, increasing GH levels may consequently reduce ASCVD risk score. Analysis of two Phase III trials of the GH-releasing hormone (GHRH) analogue tesamorelin, which can significantly reduce VAT in PWH, was also presented at IDWeek 2024. A significant overall trend in 10-year ASCVD risk score reduction was shown in tesamorelin-treated participants, around half of which were already taking lipid lowering therapies. This suggests a benefit of targeting and reducing EVAF to further impact ASCVD risk.
Introduction
The advent and increased availability of ART has led to dramatic reductions in HIV-related deaths for PWH,1,2 leading to significantly greater life expectancy.3 Modern ART is more potent, has lower adverse event profiles, and is easier to take than early treatments.4,5 However, despite these advances promoting increased rates of viral suppression, PWH have an increased risk of cardiovascular (CV)-related diseases compared with the general population, even after excluding traditional risk factors such as age, BMI, diabetes, substance abuse, and hypertension.6 For example, in PWH, increased incidences have been shown of subclinical coronary plaques,7 coronary artery disease,8 myocardial infarction,9 and heart failure.10,11
EVAF, a key characteristic of central adiposity, has been independently associated with increased CVD risk in men with HIV.12,13 While CV status may be partly attributable to HIV-associated factors,10,14 there is also evidence that links CVD risk to several classes of ART, including protease inhibitors, integrase strand transfer inhibitors, and some nucleoside analogue reverse transcriptase inhibitors.14-18 As such, 2022 International Antiviral Society (IAS)-USA Panel guidelines highlighted the need to regularly assess weight and anthropometric measurements, including waist circumference, and screen for diabetes and CVD risk in PWH taking ART, as well as to encourage CVD-targeting lifestyle changes.19
The Visceral Adiposity Measurement and Observations Study
VAMOS was a cross-sectional, multi-center, observational study that included PWH who were virologically suppressed with ART for at least 1 year and had a BMI of 20−40 kg/m2. In the analysis presented in a poster at IDWeek 2024, the investigators examined whether EVAF impacted traditional CVD risk factors and overall CVD risk in PWH in the modern ART era.20
EVAF, defined here as a VAT surface area ≥130 cm2, was quantified via an abdominal CT scan at the L4−5 vertebrae. Percentage Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), triglyceride (TG) to high density lipoprotein (HDL) ratio (TG:HDL; an alternative measure of insulin resistance), and GH levels were also analyzed. Participants completed study visits, assessments, and CT scans following overnight fasting.20
At the time of analysis, 170 participants had paired CT scans and laboratory measurements. Overall, the mean (standard deviation [SD]) VAT surface area was 229.0 (83.50) cm2 for the EVAF group (n=98) and 82.7 (28.99) cm2 in the non-EVAF group (n=72). Significant differences were shown between the EVAF group and the non-EVAF group in respective mean (SD) age (57 [9.3] versus 49 [11.4] years; p<0.0001), sex (93.9% versus 83.3% male; p=0.027), and race (79.4% White, 16.5% Black versus 59.2% White, 32.3% Black; p=0.017).
Most notably in this analysis, significant differences were shown between the EVAF and non-EVAF groups in mean (SD) 10-year ASCVD risk (20% [17.8] versus 12% [13.5], respectively; p=0.0001; Figure 1A), as well as lifetime ASCVD risk (51% [15.1] versus 43% [16.8], respectively; p=0.0166). As can be seen from Figure 1B, where the participants were analyzed as a whole, there was significant positive correlation between percentage 10-year ASCVD risk and VAT surface area.20
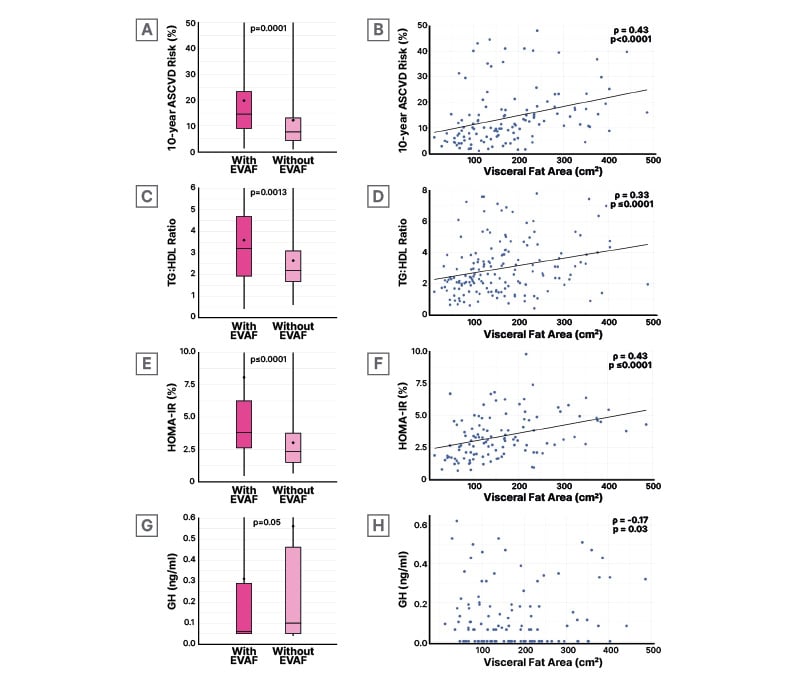
Figure 1: A) Mean (circle) and median (line) percentage 10-year ASCVD risk, C) TG:HDL ratio, E) percentage HOMA-IR, G) and growth hormone levels in participants with or without excess visceral abdominal fat; visceral adipose tissue surface area (denoted visceral fat area) correlation with B) percentage 10-year ASCVD risk, D) TG:HDL ratio, F) percentage HOMA-IR, and H) growth hormone levels.
Wilcoxon Two-Sample tests were performed for dichotomous EVAF (EVAF as Visceral Fat Area ≥130 cm2 (1A,1C, 1E, 1G). Spearman correlations were performed to determine the associations between Visceral Fat Area measurements and 10-year ASCVD risk (1B), measures of insulin resistance (1D, 1F), and GH (1H).
ASCVD: atherosclerotic cardiovascular disease; EVAF: excess visceral abdominal fat; GH: growth hormone; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; TG-HDL: triglyceride-high density lipoprotein.
The 10-year ASCVD risk score is based on several components, one of which is HDL, with an optimal level (for calculation of this score) of ≥50 mg/dL.21 Here, mean (SD) HDL in both groups was below this optimum and significantly lower in the EVAF group (46 [16.5] mg/dL) compared with the non-EVAF group (49 [13.0] mg/dL; p=0.015). Though not a direct component of the ASCVD risk score, mean (SD) TG levels were also significantly different between these groups, at 145 (68.4) mg/DL in the EVAF group versus 115 (52.1) mg/DL in the non-EVAF group (p=0.0008).20
As shown in Figure 1C, TG:HDL ratio, a marker of insulin resistance, was significantly higher in participants with EVAF and was positively correlated with VAT surface area (Figure 1D).20 These results reflect similar findings from a 2023 study of PWH, where VAT correlated with TG and HDL levels. It was also higher in PWH with detectable coronary artery calcium and presence of any coronary plaque, compared to those without such findings.22
Significant differences were also shown between participants in regard to insulin resistance measured via HOMA-IR (Figure 1E), again with a positive correlation to VAT surface area (Figure 1F). The TG:HDL and HOMA-IR findings are reflected in another ASCVD risk factor component, diabetes history, which was shown in 6.9% of the non-EVAF group but a significantly higher 19.4% of the EVAF group (p=0.021).20 These findings are of interest as there is a known association between insulin resistance and VAT in PWH,23 which may due to HIV itself,16 metabolic syndrome, and some classes of ART.24
A further ASCVD risk score component is total cholesterol (TC), with an optimum level ≤170 mg/dL.21 While this optimum was seen in the non-EVAF group (mean 167 [41.7] mg/dL), levels were elevated in the EVAF group (mean 182 [38.2] mg/dL), with a significant difference between groups (p=0.017).20 While another component that impacts ASCVD risk is current smoking status,21 in this cohort, although smoking rates were higher in the EVAF group, they were not significantly different than the non-EVAF group.20
Systolic blood pressure also contributes to ASCVD risk score, where the optimal untreated level is ≤110 mmHg.21 Both groups were above this optimal level, at 125 [14.6] mmHg in the EVAF group versus 120 [14.0] mmHg in the non-EVAF group, and it was significantly higher in the former (p=0.014). Reflecting this, while 52.0% of the EVAF group were being treated for hypertension, this was only seen in 34.7% of the non-EVAF group (p=0.025).20
The study also found significantly lower GH levels in the EVAF group (Figure 1G) with this measure inversely correlating with increasing VAT surface area (Figure 1H). The relevance of this is discussed below.20
Overall, the authors concluded that these data demonstrate how EVAF and associated factors contribute to a heightened risk of CVD in PWH on modern ART regimens.20
The Impact of Tesamorelin on Cardiovascular Risk Prediction Scores
Normally GH works to regulate vascular health, body fat distribution, and lipid metabolism. However, GH deficiency is associated with increased central adiposity, higher TG levels and hypertension rates, and lower HDL levels. Further, reductions in GH related to obesity are significantly associated with CVD risk.25
In the VAMOS findings reported above, GH levels were decreased in PWH with EVAF.20 These results bring relevance to the findings from a 2001 study in males with HIV that showed that mean GH concentrations were significantly lower, and mean VAT significantly higher, in individuals with HIV-associated lipodystrophy compared to controls and to individuals with HIV but without HIV-associated lipodystrophy. VAT was a significant predictor of mean GH concentration.26
GHRH is a hypothalamic peptide that increases pituitary GH secretion. Administration of GHRH in a study of males with HIV and lipodystrophy was shown to decrease trunk fat and improve the ratio of abdominal visceral fat to abdominal subcutaneous fat.27 Tesamorelin is a GHRH analogue that has been shown to significantly reduce VAT in PWH compared to placebo in randomized controlled trials.28-30 Significant reductions were also shown in TC and TG levels, with little effect on glucose levels or subcutaneous or limb fat.28-31
A sub-analysis of two Phase III trials of tesamorelin was presented at IDWeek 2024. This aimed to determine the impact of tesamorelin on 10-year ASCVD risk scores.32 Key inclusion criteria for trial participants were PWH with CD4 cell counts >100 cells/mm3; viral load <10,000 copies/mL; in receipt of ART for ≥8 weeks; and presence of excessive accumulation of abdominal fat (measured as waist circumference >95 cm in males and >94 cm in females, and waist to hip ratio >0.94 in males and >0.88 in females). Key exclusion criteria included fasting glucose >150 mg/dl (8.33 mmol/L), fasting TG >0.99 g/dl (11.3 mmol/L), Type 1 or Type 2 diabetes requiring medication, and untreated hypertension (systolic pressure >140 mmHg, diastolic pressure >90 mmHg).28,30,31 Participants were 85% male, 76% White, and 13% Black, with a median age of 47 years. Median BMI was 28.4 kg/m2, VAT was 168 cm2, TC was 188 mg/dL, and HDL was 43 mg/dL. Lipid-lowering medication was being taken by 44% of participants, 36% were taking blood pressure medication, and 17% had impaired glucose tolerance or diet-controlled diabetes. Baseline characteristics were similar between participants randomized to either 2 mg/day tesamorelin (n=543) or placebo (n=263) for 26 weeks.32
Baseline calculation of 10-year ASCVD risk using American College of Cardiology (ACC)/American Heart Association (AHA) criteria21 revealed that, while overall 57% of participants had low CV risk classification, almost 28% had intermediate or high risk. Similar classifications were seen using Framingham criteria, where overall 53% had low risk, with 47% having moderate or high risk (Figure 2).32
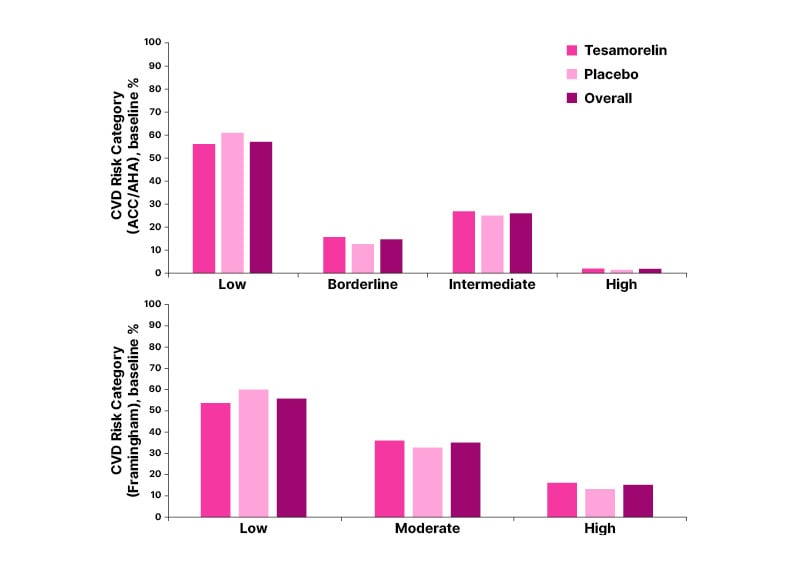
Figure 2: Cardiovascular risk classifications of study participants at baseline.
ACC/AHA: American College of Cardiology/American Heart Association; CVD: cardiovascular disease.
An effects analysis was conducted to characterize the significance of treatment impact via intermediate variables that factor into ASCVD risk prediction changes. Modifiable variables included systolic and diastolic blood pressure, TC, HDL, and low-density lipoprotein.32 Following 26 weeks’ treatment, tesamorelin was associated with an estimated decrease in ASCVD risk score of −0.40% (95% CI: −0.89%, 0.05%). The reduction was significantly higher among participants with higher CVD risk at baseline (p=0.038 for overall trend among all group participants; Figure 3).32
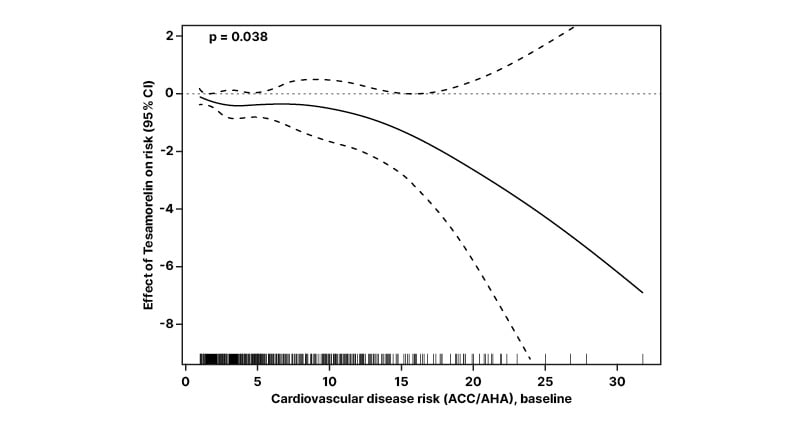
Figure 3: Estimates of the effect of tesamorelin on average cardiovascular risk score, calculated using 10-year atherosclerosis cardiovascular disease risk score.
Estimated effect shown as a function of baseline 10-year ASCVD risk score. Pointwise 95% CIs are displayed, along with a rug plot showing the distribution of the baseline risk score.
ACC/AHA: American College of Cardiology/American Heart Association; ASCVD: atherosclerotic cardiovascular disease.
Administration of lipid lowering therapies to PWH with low to moderate CVD risk can reduce noncalcified plaque volume and progression and potentially reduce occurrence of a major adverse CV event.33 In this analysis, 44% in the tesamorelin group and 43% in the placebo group were taking lipid-lowering therapies at baseline and remained stable through the duration of study. Of note, ASCVD risk reduction was independent of lipid lowering therapies, and it was postulated that this risk reduction was driven predominantly by reductions in TC levels, which were significant compared to placebo (p=0.061).32
The authors concluded that “this analysis provides evidence that reductions in EVAF with tesamorelin lead to a significant reduction in forecasted CVD risk in PWH even among a group heavily treated with lipid-lowering therapy.”32
Conclusion
As PWH have an increased lifetime CVD risk compared with the general population,6 addressing factors associated with this risk is key in such individuals. With VAMOS findings highlighting how EVAF may contribute to heightened CVD risk in PWH,20 attention should be given to targeting EVAF when considering CVD risk management. Therapeutic tools, such as tesamorelin, have been shown to significantly reduce EVAF in PWH and may also be associated with decreased ASCVD risk scores.2







