Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a new pathogen that was responsible for the global pandemic that started in Wuhan, China in 2019. It causes COVID-19, manifesting as viral pneumonia with concomitant acute respiratory failure and, in certain cases, multiorgan failure and death.
Kidney involvement is common and can be aetiologically heterogeneous. Acute kidney injury is mostly caused indirectly, especially in the context of systemic inflammation, hypoxaemia, hypotension, shock, and increased oxidative stress. Complement activation, tubulointerstitial damage, and endothelial dysfunction with resultant thromboses are also important factors in kidney injury. Histologically, SARS-CoV-2 was found to induce predominant tubulointerstitial changes and in some cases, glomerular changes. In a certain subgroup of patients with the APOL1 high-risk allele variant, a collapsing glomerulopathy, similar to HIV-associated nephropathy, was found. This entity was later named COVID-19-associated nephropathy. In this article, the authors present the pathophysiology behind SARS-CoV-2-related kidney involvement and the development of COVID-19-associated nephropathy.
Key Points
1. Kidney injury in COVID-19 is common and multifactorial, occurring via several mechanisms including indirect damage in the context of systemic inflammation, hypoxaemia, hypotension, shock, and increased oxidative stress; complement activation; tubulointerstitial damage; and endothelial dysfunction with resultant thromboses.2. A subgroup of patients with an APOL1 high-risk allele variant have been found to have a collapsing glomerulopathy with podocyte proliferation, with or without severe acute respiratory syndrome coronavirus 2 viral inclusion particles; this entity has been named COVID-19-associated nephropathy (COVAN).
3. Supportive treatment is utilised for cases of COVAN; further studies are needed to improve the recognition of these patients and to develop optimal treatment strategies.
INTRODUCTION: RECENT EPIDEMIOLOGIC DATA AND GLOBAL BURDEN OF COVID-19
In 2019, a cluster of unusual pneumonia cases was identified in Wuhan, China. The patients presented with acute respiratory distress syndrome, which resulted in respiratory failure and, in some cases, multiorgan failure and death. The disease spread throughout China and a new coronavirus, which was designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of the epidemic. The resultant disease was named COVID-19, and it quickly spread worldwide, causing a global pandemic that is still currently ongoing.1
Since February 2022, over 350 million cases and 5.5 million deaths attributed to COVID-19 have been identified worldwide. The countries with the most confirmed cases and deaths are the USA, India, France, and Italy.2 Due to how quickly the disease has spread, COVID-19 presents a major threat to health systems worldwide, making it one of the most prominent infectious diseases of this century.1
Studies have shown that COVID-19 more commonly and severely affects obese patients and those with chronic illnesses, including diabetes mellitus, arterial hypertension, and chronic kidney disease (CKD).3 Additionally, a direct causal link between infection with SARS-CoV-2 and organ damage has been found, resulting in new pathological and clinical entities, which have thus far not been described.4
In this article, the authors present COVID-19-associated nephropathy (COVAN), a new variant of collapsing focal segmental glomerulosclerosis (FSGS) that has been attributed to infection with SARS-CoV-2.4
PATHOPHYSIOLOGY: THE EFFECTS OF SARS-COV-2 ON THE KIDNEY
Several studies and reports have shown that angiotensin-converting enzyme 2 (ACE2) is the host cell receptor for SARS-CoV-2.5 ACE2 is an enzyme that is expressed in various human tissues, especially in the kidney, gastrointestinal tract, lungs, heart, and testes, indicating that these tissues are most susceptible to infection with the virus.6
ACE2 is a powerful negative regulatory peptide of the renin-angiotensin aldosterone system, as it can cause the degradation of angiotensin II and therefore lead to vasodilatation, suppression of inflammation, reduction in oxidative stress, and cell apoptosis. The infection with SARS-CoV-2 causes a downregulation of circulating ACE2 and a substantial increase in the level of angiotensin II, which is a crucial component in the cytokine storm and proinflammatory state that is often observed in patients with COVID-19.7,8 A peptidase-independent, receptor-like function is also important in SARS-CoV-2 infection. Studies have determined that the interaction between the S1 subunit of the SARS-CoV-2 spike protein and ACE2 leads to membrane fusion between the virus and target cells, increasing the spike protein-driven viral infection. Furthermore, endocytosis may be another route by which viral entry and cell infection can take place.9 During cellular entry, the internalisation of ACE2 leads to an increase in angiotensin II activity, thereby causing vasoconstriction, inflammation, increased oxidative stress, and cell death.7 The kidney is one of the organs with the highest ACE2 expression and activity, especially in the podocytes, mesangial cells, and proximal tubular cells, making it extremely susceptible to SARS-CoV-2 infection.10
Renal damage in patients with SARS-CoV-2 is multifactorial. Firstly, indirect injury of the renal parenchyma is common due to systemic inflammation, hypoxaemia, hypotension, shock, and increased levels of reactive oxygen species.11 Secondly, complement activation, tubulointerstitial damage, and endothelial dysfunction with resulting vascular thromboses also contribute to kidney injury.11 Kidney biopsy results have shown tubulointerstitial oedema, diffuse acute proximal tubular injury with the loss of brush border, protein and pigment casts in the tubular lumen, and diffuse erythrocyte aggregation in the peritubular and glomerular capillary loops. The observed glomerular lesions have been minor, with only modest endothelial swelling and non-specific immunofluorescence staining.12 Interestingly, podocytes showed vacuolation and detachment from the glomerular basement membrane. Some studies have later reported the presence of a severe, collapsing glomerulopathy in the kidneys of patients of African descent with SARS-CoV-2 infection, mimicking the findings that were seen in patients with HIV-associated nephropathy.13 The term COVAN was coined, representing a specific form of collapsing FSGS in patients with COVID-19.14
COVID-19-ASSOCIATED NEPHROPATHY (COVAN)
Collapsing glomerulopathy is a severe, aggressive, and distinct histological variant of FSGS, characterised by segmental or global glomerular tuft collapse with hypertrophy and hyperplasia of the overlying podocytes. Tubulointerstitial involvement, including acute tubular injury, tubular dilatation, and interstitial inflammation, is also a common finding.15,16
Weiss et al.16 were the first to describe collapsing glomerulopathy as a clinical and pathological entity in 1986, when they presented a case series of six Black patients with proteinuric kidney impairment and glomerular collapse following a brief period of febrile illness. The series suggested that this may have been the result of a potential infectious causative agent, although no microorganism was identified.16 Soon after, a similar clinical and histological entity was confirmed in patients with HIV.17
In general, collapsing glomerulopathy can be primary or secondary in association with different clinical entities, including malignancies, thrombotic microangiopathy, sickle cell disease, cholesterol embolisation, genetic mutations, drugs (e.g., pamidronate, interferon), autoinflammatory conditions (e.g., systemic lupus erythematosus, haemophagocytic syndrome), and viral infections such as HIV, parvovirus B19, cytomegalovirus, Epstein–Barr virus, and, in recent times, SARS-CoV-2 (Table 1).18
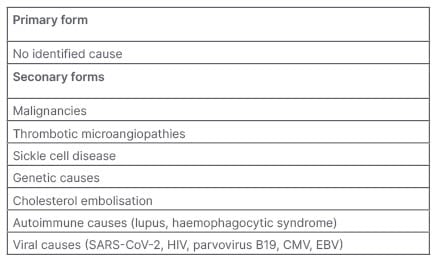
Table 1: Different forms of collapsing variant of focal segmental glomerulosclerosis.
CMV: cytomegalovirus; EBV: Epstein–Barr virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2.
Several case reports on COVAN have been published so far. Larsen et al.19 reported a case of a 44-year-old female of an African American background who presented to the emergency department with fever, cough, vomiting, and flank pain. An acute kidney injury with haematuria and proteinuria was found, and an infection with SARS-CoV-2 was confirmed. Immunologic tests were negative. Due to worsening kidney function, renal replacement therapy with dialysis was started, and later on, a kidney biopsy was performed. Histological analysis showed a collapsing variant of FSGS with epithelial cell hypertrophy and signs of proximal tubular cell injury. No definitive viral inclusion particles were identified by electron microscopy. APOL1 genotyping on the biopsy material was performed and the patient was found to be homozygous for the G1 risk allele. In the follow-up period, the patient’s clinical status markedly improved, although they remained dialysis-dependent.19
In a report by Peleg et al.,13 a case of a 46-year old male from a West-African background with severe kidney injury was presented. The patient complained of sore throat, malaise, fever, and vomiting 3 weeks before admission. Laboratory tests were notable for severe acute kidney injury with nephrotic-range proteinuria, hypoalbuminaemia, elevated lactate dehydrogenase, and elevated inflammatory markers. Hepatitis, HIV markers, and immunology were all negative. The patient was started on haemodialysis and, later on, a kidney biopsy was carried out. The biopsy findings were consistent with collapsing glomerulopathy with signs of mild to moderate tubulointerstitial oedema and arteriolosclerosis. Genotyping for the presence of APOL1 high-risk alleles (G1 and G2) revealed that the patient was homozygous for the G1 allele. Due to respiratory symptoms and elevated inflammatory markers, a nasal swab for SARS-CoV-2 was performed, and it was positive. Later on, in-situ hybridisation for SARS-CoV-2 was performed on kidney tissue, which came back negative. No viral inclusion particles were detected by electron microscopy.13
Kissling et al.20 presented a case of a 63-year-old Black male who was admitted due to SARS-CoV-2 pneumonia. In the first few days of hospitalisation, they developed a rapidly progressive acute kidney injury with nephrotic-range proteinuria (5 g/L). The patient’s kidney biopsy showed collapsing glomerulopathy with acute tubular necrosis. In this case, electron microscopy showed several viral inclusion particles in the podocytes, consistent with SARS-CoV-2. Further work-up showed that the patient was homozygous for the APOL1 G1 high-risk allele. The patient’s kidney function improved later on and they did not need renal replacement therapy.20
Magoon et al.21 presented two cases of collapsing FSGS in patients with COVID-19. A 28-year-old female of an African American background presented to the emergency department with fever, fatigue, shortness of breath, and cough. The patient had normal creatinine and nephrotic proteinuria at admission. Due to the clinical picture and bilateral opacities on the chest X-ray, a nasal swab for SARS-CoV-2 was performed and it came back positive. In the next 4 days, their creatinine level started to rise, and they were put on haemodialysis on Day 7. A kidney biopsy was performed to determine the cause of proteinuric acute kidney injury. A collapsing variant of FSGS was found and no viral inclusion particles were detected with electron microscopy. All of the other work-up, hepatitis and HIV markers, and immunology came back negative. The patient was later found to be homozygous for the APOL1 G1 high-risk allele. They remained dialysis-dependent as their kidney function did not recover.
In the second case, a 56-year old African American male with SARS-CoV-2 infection and acute worsening of CKD and proteinuria was presented. Similar to the previous case, their kidney biopsy showed severe collapsing glomerulopathy with podocyte hypertrophy, and the electron microscopy did not show viral inclusion particles in this case either. The patient was found to be heterozygous for APOL1 G1 and G2 alleles. Their kidney function did, however, improve, and they did not need dialysis after they were discharged from the hospital.21
Kudose et al.22 performed a study on longitudinal outcomes of patients with COVAN and other associated podocytopathies. Out of the 23 patients with COVAN that were included, 21 of them (91%) were Black and two (9%) were White. Most of the patients had the high-risk APOL1 genotype. According to their findings, 50% of the patients remained dialysis-dependent after hospital discharge.22
The most important characteristics of COVID-19-associated collapsing glomerulopathy were gathered by the authors of this paper (Table 2). Judging by the case reports presented thus far, the APOL1 high-risk genotype is of crucial significance in the development of COVAN. Due to the greater prevalence of high-risk APOL1 alleles in patients with an African American heritage, COVAN is more common in these patients, with a few cases described in White patients as well.22 The authors found no data suggesting the presence of COVAN in other ethnicities and/or races. In an experimental model of a ‘two-hit’ hypothesis, the APOL1 risk variant acts as a ‘first hit’ and the infection with a virus (such as SARS-CoV-2) acts as a ‘second hit’ through cytokine and chemokine release, ultimately resulting in podocyte dedifferentiation, proliferation, and hypertrophy.18,23

Table 2: Crucial characteristics of COVID-19-associated collapsing glomerulopathy.
In majority of the cases, viral inclusion bodies were not detected on renal histology. Immune system dysregulation with consequent cytokine release, and not the viral presence per se, is most likely the trigger that leads to impairment in glomerular cell autophagy, mitochondrial function, and cell injury.4,24 It appears that even anti-SARS-CoV-2 vaccines can lead to immune system activation and collapsing glomerulopathy in at-risk patients.25
Patients with homozygosity for APOL1 risk alleles would likely benefit from prompt anti-viral and/or anti-inflammatory treatment to prevent the cytokine storm and the development of kidney impairment. Further studies are needed to determine the role of APOL1 genotyping in certain patient groups in order to employ an optimal treatment strategy.24
CONCLUSION
COVAN is a new and emerging clinical entity, related to the SARS-CoV-2 infection. It can present as acute kidney injury or acute worsening of pre-existing CKD with marked proteinuria, usually in the nephrotic range. The COVAN pathophysiological features are associated with APOL1 high-risk genotype, often found in patients with African heritage. Kidney biopsy shows collapsing glomerulopathy with podocyte proliferation, with or without SARS-CoV-2 viral inclusion particles. Treatment is supportive. Further studies are needed to improve the recognition of these patients and to develop optimal treatment strategies.








