Abstract
Renal involvement with rapidly progressive glomerulonephritis is a common manifestation of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides characterised by end-stage renal disease and high mortality rates in untreated and late referral patients. Long-term renal survival has improved dramatically since the addition of cyclophosphamide and, more recently, rituximab in association with corticosteroids to remission induction therapeutic regimens. However, renal prognosis remains unfavourable for many patients and mortality is still significantly higher than in the general population. In this review, the open challenges to be addressed to optimise remission induction therapy, especially in patients with advanced kidney failure, are analysed. This concerns the first-line therapy (cyclophosphamide or rituximab) based on different parameters (estimated glomerular filtration rate at baseline, new or relapsed disease, ANCA specificity, tissue injury, and safety) and the role of plasma exchange. Furthermore, the paper discusses future perspectives on induction remission therapy by reporting recent advances in new targeted therapies, with particular reference to avacopan, an orally administered selective C5a receptor inhibitor.
INTRODUCTION
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) include granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA).1 These autoimmune disorders can affect any organ system, but the kidneys are often involved. Renal involvement ranges between 71% and 88% in patients with GPA and MPA,2 whereas in EGPA it occurs in up to 25% of cases.3 Severe renal involvement is an uncommon finding in cases of EGPA;4 therefore, this review is focussed exclusively on GPA and MPA as two clinical conditions with major renal involvement.
Prior to the introduction of cyclophosphamide (CYC)-based regimens in the late 1970s, the 2-year survival rate of AAV patients was approximately 20%.5 Standard immunosuppression with CYC and gradually tapered corticosteroids (CCS) for remission induction therapy have dramatically improved the prognosis of the disease in patients with generalised or severe GPA or MPA, with overall remission rates usually exceeding 90% and 5-year patient survival rates as high as 80%.5 However, this treatment is associated with significant toxicity, including an enhanced risk of infection, myelosuppression, infertility, malignancy, and cardiovascular disease.6
Consequently, there has been a growing impetus to look for a new, less toxic, and more specific treatment for patients with generalised and severe disease. Two randomised controlled trials (RAVE7 and RITUXVAS8) found that rituximab (RTX), a B cell-depleting agent, is as effective as CYC for induction of remission in patients with newly diagnosed GPA and MPA. The RAVE trial7 also demonstrated the superiority of RTX versus CYC in patients with relapsing disease. Nevertheless, in both trials the short-term adverse event rate after RTX treatment was not lower than of CYC.
Despite this advancement and the enrichment of therapeutic armamentarium, the management of remission induction in patients with AAV and renal involvement continues to challenge nephrologists because renal prognosis is still unfavourable and a significant proportion of patients (20–25%) develop end-stage renal disease (ESRD) within a few years of diagnosis.9
This review will discuss the remaining challenges in the management of remission induction in AAV with renal involvement, answering crucial questions and presenting recent advances in novel targeted therapies and treatment strategies that may further help to modify the disease course, thereby leading to improved renal outcomes and patient survival.
SHOULD ALL PATIENTS WITH RENAL INVOLVEMENT BE TREATED WITH RITUXIMAB, EVEN IF THEY HAVE ADVANCED RENAL FAILURE?
What the Results of Current Studies Tell Us About the Renal Outcomes
RTX was initially used in open-label trials of patients with refractory or relapsing GPA and MPA and demonstrated clinical remission rates in approximately 90% of cases within 6 months.10-14 However, these preliminary studies did not include patients with severe renal involvement. The RAVE trial7 enrolled 197 patients, of whom approximately half had significant renal disease defined by the presence of at least one of the following findings at baseline: active, biopsy-proven, pauci-immune glomerulonephritis; red blood cell casts on urine microscopy; and/or increase in serum creatinine >30% or a >25% decrease in creatinine clearance. Although in patients with significant renal disease, the baseline mean estimated glomerular filtration rate (eGFR) was worse in the RTX group (41 versus 50 mL/min per 1.73 m2; p=0.05), a post hoc analysis of the trial showed that RTX was as effective as oral CYC in this subgroup.15 The proportion of patients who achieved complete remission at 6 months was not significantly different between the two treatment groups (RTX: 61% versus CYC: 63%) and there was no difference in the proportion of patients with sustained remission at 18 months (RTX: 75% versus CYC: 76%).15 The latter finding is significant because, in order to achieve remission at 3–6 months, a maintenance regimen with azathioprine (AZA) was administered only to patients in the CYC group, whereas those in the RTX group received no further therapy.7,15 Mean eGFR also increased similarly in both groups when patients were stratified by baseline eGFR, even among those with an eGFR <30 mL/min per 1.73 m2.15 These data represent the strengths of the RAVE trial7 in supporting the use of RTX in patients with major renal involvement; however, a limitation of the RAVE trial was the exclusion of patients with advanced renal failure (serum creatinine >4 mg/dL), as the clinical evidence was not sufficient to suggest their inclusion in an investigational treatment study at the launch of the trial. Therefore, the authors concluded that additional studies were required to understand the applicability of RTX for patients with advanced kidney failure.15
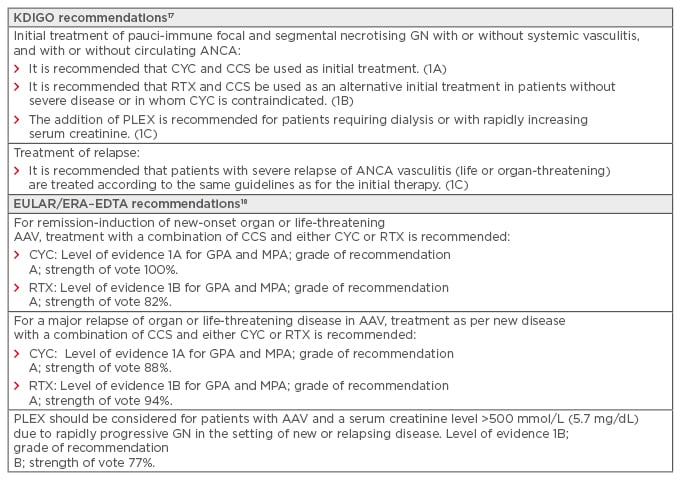
In contrast with the RAVE trial, RITUXVAS8 enrolled 44 patients newly diagnosed with GPA and MPA with severe renal disease (median eGFR: 20 mL/min per 1.73 m2), also including patients requiring dialysis at trial entry. The participants were randomised in a 3:1 ratio to receive either RTX plus CCS without further maintenance treatment or intravenous CYC for 3–6 months plus CCS followed by AZA in the maintenance phase. At 12 and 24 months, there was no difference in the proportion of sustained remission and ESRD between the RTX and CYC groups.8,16 However, the weakness of the RITUXVAS trial, beyond the small number of participants, was that patients in the RTX group also received two concomitant pulses of intravenous CYC, and, for those with progressive disease within the first 6 months, a third dose of intravenous CYC was allowed, making it very difficult to discern the specific contribution of CYC in the RTX-treated patients. Based on these data, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines17 recommend the use of RTX plus CCS as an alternative initial treatment only in patients without severe renal disease or in whom CYC is contraindicated (Box 1).
Box 1: Current guidelines for remission induction therapy for antineutrophil cytoplasmic antibody-associated vasculitides with severe renal involvement.
AAV: antineutrophil cytoplasmic antibody-associated vasculitides; ANCA: antineutrophil cytoplasmic antibody; CCS: corticosteroids; CYC: cyclophosphamide; ERA–EDTA: European Renal Association–European Dialysis and Transplant Association; EULAR: European League Against Rheumatism; GN: glomerulonephritis; GPA: granulomatosis with polyangiitis; KDIGO: Kidney Disease Improving Global Outcomes; MPA: microscopic polyangiitis; PLEX: plasma exchange; RTX: rituximab.
Recently, two retrospective multicentre studies evaluating the efficacy of RTX plus CCS without concomitant CYC in patients with severe renal involvement have achieved high rates of remission and dialysis independence.19,20 However, these studies have limitations due to their retrospective designs and small sample sizes. Further prospective randomised trials are needed to confirm these findings in this subset of patients.
In 2016, the 2009 European League Against Rheumatism (EULAR) recommendations for the management of ANCA-associated vasculitis were updated by the European Renal Association–European Dialysis and Transplant Association (ERA–EDTA).18 For remission induction of new-onset or major relapse of organ or life-threatening GPA and MPA, treatment with a combination of CCS and either CYC or RTX is now recommended. The grade of recommendation was A for both CYC and RTX but with a different level of evidence 1A for CYC and 1B for RTX, confirming the need for further evidence in this field (Box 1).
What is the Impact of Renal Tubular Lesions on Therapeutic Choice?
The evaluation of histological parameters along with clinical parameters could also be relevant in determining therapeutic choice. Among patients in the RITUXVAS trial, both B cell and T cell-mediated tubulointerstitial lesions were present in renal biopsies before treatment with RTX. However, only tubular intraepithelial T cells were predictive of impaired renal function during follow-up. The analysis of patients treated with CYC, an immunosuppressive agent directed towards both B cells and T cells, did not show any evidence that T cell tubulitis was related to renal outcome.21 These data raised the question of whether T cell tubulitis represents a negative predictor for all treatments or whether its predictive significance is limited to RTX due to undertreatment of T cell-mediated lesions by B cell-depleting agents.
Recently, Geetha et al.22 conducted a similar study using renal biopsies from patients who participated in the RAVE trial. In contrast to the results of the RITUXVAS study, this study showed that interstitial B and T cell infiltrates had no significant impact on long-term prognosis, regardless of the immunosuppression regimen used (RTX or CYC).22
Repeat renal biopsies in future trials would help to clarify these contradictory results and identify the extent of B and T cell infiltration, which could potentially be a significant clinical factor in determining the adequate therapy for individual patients to ensure that active lesions are adequately treated.
What is the Impact of Safety and Adverse Events on Therapeutic Choice?
Given that safety is a key concern in the evaluation of immunosuppressive agents, there may be individual clinical situations in which RTX is more appropriate than CYC. These may include patients with a high cumulative dose of CYC due to previous exposure, those with a history of malignancy, and those who are of childbearing age but do not yet have any offspring. However, RAVE7 and RITUXVAS8 did not show any benefit of RTX in terms of incidence of adverse events, regardless of severity.23 Particularly, the incidence of severe infections was considerable in the RAVE and RITUXVAS trials (12% and 18%, respectively) and did not differ between the CYC and RTX-based induction treatments. Initial renal dysfunction determined by eGFR was also associated with a higher risk of subsequent infection for both treatment groups.
Although using RTX in patients with severe infection has been reported as an efficacious remission induction treatment,24 this is not recommended because moderate-to-severe hypogammaglobulinaemia occurs in >50% of AAV patients treated with RTX, resulting in an increased risk of infections that could be even higher in those with reduced renal function.25 Hypogammaglobulinaemia with an early onset is usually transient and benign,26 whereas hypogammaglobulinaemia with a late onset is commonly severe and associated with infection.27 Late-onset neutropenia (LON), defined as an absolute neutrophil count <1.0×109 for >1 month after the last RTX infusion with spontaneous recovery when other causes are ruled out, has also been described in both GPA and MPA, and has been reported to be associated with a high incidence of infections. For example, in a recent single-centre analysis of 59 patients with AAV, LON developed in 12% of patients.28
Uncommon but serious adverse events after RTX treatment include hepatitis B reactivation, which is largely preventable with antiviral prophylaxis, and progressive multifocal leukoencephalopathy, caused by reactivation of the human John Cunningham polyomavirus.29-31 However, progressive multifocal leukoencephalopathy and hepatitis B reactivation have also been reported in patients with GPA treated with CYC.32,33
The malignancy incidence in patients treated with RTX or CYC has been recently investigated in a retrospective study of 323 patients with AAV.34 During a mean follow-up of 5.6 years, patients treated with RTX did not show an increased risk compared with the general population. In contrast, patients treated with CYC had a 4.61-fold higher risk of developing malignancies than those treated with RTX. Longer follow-up studies are now required to validate these data.
THERAPEUTIC APPROACH: PATIENTS WITH NEWLY DIAGNOSED ANTINEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIDES AND THOSE WITH DISEASE RELAPSE
The therapeutic approach to relapse in patients with GPA or MPA and renal involvement depends on the degree of severity and whether the patient is still undergoing treatment with a maintenance immunosuppressive regimen at the time of relapse.
Treatment of Mild Renal Relapse
Patients with mild, non-organ-threatening relapse (e.g., recurrent red blood cell casts on urine microscopy without concomitant increase in serum creatinine) who are still undergoing maintenance therapy can initially be treated by increasing the dose of CCS and of the respective immunosuppressive agent used for maintenance therapy, if the relapse occurs during a reduction in dose of maintenance therapy.18,35 Mild, non-organ-threatening relapses that arise after discontinuation of maintenance therapy can be treated with the resumption of the prior maintenance therapy. In the latter case, the maintenance therapy should be continued for a more extended period than planned before the relapse.18 If the nephrologist is uncertain that the relapse is mild, a kidney biopsy must be performed to clarify whether the repeat-induction therapy is warranted. In patients with multiple mild relapses, B cell depletion with RTX must be considered as an alternative approach.35 Most recommendations for non-severe relapses come from the 2016 EULAR/ERA–EDTA guidelines for the management of AAV; however, these recommendations do not provide a level of evidence or a grade of recommendation.18
Treatment of Severe Renal Relapse
The 2016 EULAR/ERA–EDTA recommendations for the management of AAV suggest that severe relapses should be treated with the resumption of induction therapy using a CYC-based or RTX-based regimen (CYC: level of evidence 1A; Grade of recommendation A versus RTX: level of evidence 1B; Grade of recommendation A).18 In patients who relapse after successfully achieving remission with a CYC-based regimen, RTX is preferred because the cumulative dose of CYC is associated with significant toxicity.18,33 RTX is also the therapy of choice for patients who relapse after previously achieving remission with RTX-based therapy.18,36
The best data in patients with relapsing GPA and MPA come from the RAVE trial. The rate of remission induction in patients with relapse was higher with RTX at 6 and 12 months, but not at 18 months.7,23 RTX and CYC followed by AZA achieved similar remission rates at 18 months, although patients in the RTX group who achieved a complete remission by 6 months received no additional immunosuppression for >1 year.23 However, CYC-based therapy should be considered for patients whose relapse is characterised by advanced renal failure, as it is for patients with newly diagnosed AAV, for the reasons mentioned above (Box 1).
What is the Impact of Antigenic Specificity of Antineutrophil Cytoplasmic Antibodies on Therapeutic Choice?
To date, there is growing evidence that ANCA specificity is superior to clinical diagnosis in defining homogeneous groups of patients, since proteinase 3 (PR3)-ANCA and myeloperoxidase (MPO)-ANCA are associated with different genetic backgrounds and epidemiologic patterns.37 Data from most cohorts show that patients with MPO-ANCA have poorer renal outcomes than those with PR3-ANCA.38-40 However, regarding the frequency of relapses, numerous studies have shown that these are much more frequent in patients with PR3-ANCA seropositivity.2,38,41
In the RAVE trial,7 at the 6-month timepoint, significantly more patients became PR3-ANCA negative after RTX therapy than after CYC with AZA therapy (50% versus 17%), whereas comparable proportions of patients receiving each therapy became MPO-ANCA-negative. Most importantly, a post hoc analysis of the RAVE trial showed a similar ratio of complete remission at 6 months in both treatment groups among the subgroup of patients with MPO-ANCA seropositivity, whereas RTX was significantly more effective than CYC with AZA in the subgroup of patients with PR3-ANCA (65% versus 48%).42 Moreover, among patients with PR3-ANCA who had relapsing disease at baseline, the risk of disease relapse in RTX-treated patients was inferior not only at 6 months, but also at 12 and 18 months, despite the fact that patients randomised to RTX had not received a maintenance regimen.42 However, in another post hoc analysis of patients with renal involvement enrolled in the RAVE trial, no variations in remission rates or improvements in eGFR at 18 months were observed when the analysis was stratified by ANCA type, AAV diagnosis (GPA versus MPA), or new diagnosis versus relapsing disease at entry.15
In conclusion, the demonstrated superiority of RTX compared to CYC in patients with PR3-ANCA and in those with relapsing disease has not yet been confirmed in long-term follow-up of patients with renal involvement.15 Further clinical trials are needed to evaluate this question in well-defined homogenous patient populations, according to ANCA specificity.
SHOULD ALL PATIENTS WITH ADVANCED RENAL INVOLVEMENT BE TREATED WITH ADJUNCTIVE PLASMA EXCHANGE SESSIONS?
The rationale for plasma exchange (PLEX) in AAV is that removal of ANCA and other plasma constituents involved in the pathogenesis of the disease could reduce further tissue damage and promote reversal of the pathologic process.43 The effect of PLEX in addition to standard immunosuppressive therapy in patients with AAV and renal involvement was evaluated in an initial randomised trial that demonstrated the efficacy of PLEX only in the subgroup of patients with serum creatinine ≥500 mmol/L (5.8 mg/dL) or on dialysis at diagnosis.44 In 2007, the MEPEX trial,45 the largest randomised trial in patients with severe renal disease (serum creatinine >500 mmol/L), was published. At 3 months, a significantly higher number of patients were alive and independent of dialysis in the PLEX group. Additionally, PLEX was associated with a 24% reduction in the risk of progression to ESRD at 12 months.45 A subsequent meta-analysis of 387 patients from nine trials, including the MEPEX trial, showed a 20% relative risk reduction in the composite outcome of death or ESRD requiring dialysis after the addition of PLEX to standard immunosuppressive therapy.46 However, too few patients were randomly assigned and sensitivity analyses were not sufficiently robust to reliably conclude that PLEX results in at least a moderate decrease in the composite endpoint of ESRD or death.46
Moreover, although these short-term PLEX results are encouraging, the long-term benefits remain unclear. In fact, long-term follow-up of the MEPEX trial showed an attenuated benefit of PLEX with no significant reduction of progression to ESRD at 4 years, and equivalent mortality in both groups (51%).47 Currently, the most recent EULAR/ERA–EDTA recommendations for the management of AAV suggest that PLEX should be considered for patients with AAV and a serum creatinine level >500 mmol/L in the setting of new or relapsing disease (Box 1). 18 In conclusion, PLEX continues to be a promising therapy, but further trials are required before its widespread use for patients with renal vasculitis can be implemented. The ongoing PEXIVAS trial48 should help to further clarify the value of PLEX in these patients.
NOVEL TARGETED AGENTS AND FUTURE PERSPECTIVES ON INDUCTION REMISSION THERAPY FOR ANTINEUTROPHIL CYTOPLASMIC ANTIBODY-ASSOCIATED VASCULITIDES WITH RENAL INVOLVEMENT
The greatest challenge in the management of AAV is the development of new agents and innovative strategies, which are urgently needed to improve patient prognosis and reduce the comorbidities associated with current regimens.
As previously mentioned, to date, high-dose CCS remain an integral part of induction remission therapy for AAV, in combination with CYC or RTX. Even though CCS rapidly control inflammation and prevent further renal damage, the increased susceptibility to infections and other potential comorbidities, such as diabetes, cardiovascular events, osteoporosis, cataracts, and gastrointestinal complications,49 has prompted researchers to reduce or replace their use. The aforementioned multinational PEXIVAS trial48 and the LoVAS trial50 could provide valuable information on this crucial question (Table 1).
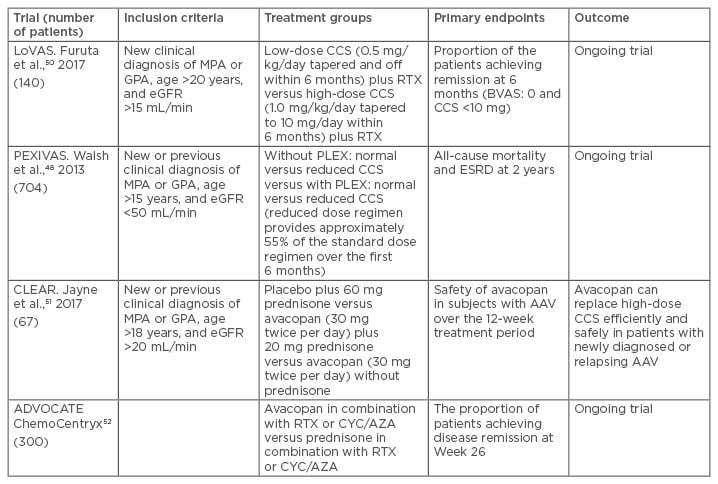
Table 1: Trials for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitides with renal involvement and corticosteroid-sparing regimens.
AAV: antineutrophil cytoplasmic antibody-associated vasculitides; AZA: azathioprine; BVAS: Birmingham Vasculitis Activity Score; CCS: corticosteroids; CYC: cyclophosphamide; eGFR: estimated glomerular filtration rate; ESRD: end-stage renal disease; GPA: granulomatosis with polyangiitis; MPA: microscopic polyangiitis; PLEX: plasma exchange; RTX: rituximab.
However, the most promising advancement for remission induction therapy is avacopan (CCX168), an orally administered selective C5a receptor inhibitor (Table 2). The efficacy of avacopan was first tested in an ANCA-associated glomerulonephritis mouse model induced by injection of MPO immunoglobulin G.62 The recently completed CLEAR study,51 a Phase II, randomised, double-blind, placebo-controlled trial, met its primary endpoint, indicating that avacopan can replace high-dose CCS efficiently and safely in patients with newly diagnosed or relapsing AAV (Table 1). The 67 enrolled patients were randomised to receive placebo plus prednisone starting at 60 mg daily (control group), avacopan (30 mg twice daily) plus reduced-dose prednisone (20 mg daily), or avacopan (30 mg twice daily) without prednisone. The early efficacy of avacopan was substantiated by a rapid improvement in albuminuria, which was statistically significantly superior to the control group. Moreover, renal inflammation improved rapidly and to a higher degree in the avacopan groups compared with the control group, as demonstrated by the greater reduction of urinary monocyte chemoattractant protein-1 levels, a renal inflammation marker, in the avacopan groups. The most important finding from the CLEAR trial regarding renal outcomes is that eGFR and haematuria improved similarly in all three groups over the 12-week treatment period, indicating that improvement in renal function in patients receiving avacopan did not require high-dose CCS. Another Phase II trial, the CLASSIC trial,53 investigated the addition of two different doses of avacopan or placebo to standard-dose CCS with CYC or RTX. No safety concerns were described in the avacopan treatment groups, and a trend towards a dose-dependent improvement in clinical responses was shown. ADVOCATE,52 a Phase III trial, is now enrolling patients and will assess the safety and effectiveness of avacopan as an alternative to prednisone in inducing and maintaining remission in patients with AAV (Table 1).
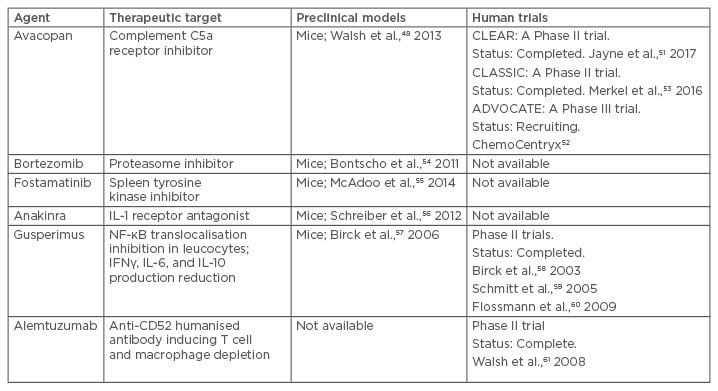
Table 2: New agents investigated in preclinical models and clinical trials in humans for antineutrophil cytoplasmic antibody-associated vasculitides with renal involvement.
IFN: interferon; IL: interleukin; NF-kB: nuclear factor kappa B.
Ofatumumab, a humanised anti-CD20 monoclonal antibody, which, like RTX, acts by depleting B cells, has been shown to be effective in the treatment of AAV patients, including those with renal involvement, thereby making this agent a possible alternative for use in patients who cannot take or tolerate RTX.63 Other new potential therapeutic agents in the induction remission therapy of AAV with major renal involvement include (Table 2):
- Bortezomib, a proteasome inhibitor, found to be more efficacious than CYC combined with CCS in decreasing the number of MPO-specific plasma cells and anti-MPO titres, thereby preventing the development of necrotising crescentic glomerulonephritis in a mouse model of MPO-AAV.54
- Fostamatinib, a selective spleen tyrosine kinase inhibitor that blocks B cell activation, shown to be an effective treatment for crescentic glomerulonephritis and lung haemorrhage in a rodent model of MPO-AAV.55
- Anakinra, a recombinant non-glycosylated human interleukin-1 receptor antagonist used in the treatment of rheumatoid arthritis, reducing the severity of necrotising crescentic glomerulonephritis in a mouse model of MPO-AAV.56
Other agents, such as gusperimus57-60,64 and alemtuzumab,61 despite having been demonstrated to be effective, should not be used in AAV due to serious adverse events (Table 2).
Belimumab, a human monoclonal antibody that inhibits B cell activating factor, also known as B lymphocyte stimulator, is currently approved for the treatment of active systemic lupus erythematosus excluding renal involvement. In AAV, the effect of belimumab in combination with AZA is currently underway as a remission maintenance strategy in the BREVAS trial,65 but its role in induction therapy, particularly in patients with renal involvement, is unknown and could be investigated in future trials in combination with standard therapy.
CONCLUSION
In recent years, RTX has enriched our armamentarium for remission induction treatment for severe organ or life-threatening AAV; however, data from randomised controlled trials on the efficacy of RTX in patients with advanced kidney failure without concomitant CYC are lacking. Additionally, despite the reported superiority of RTX in patients with relapsing disease and those with PR3-ANCA seropositivity, no differences in remission rates or increases in eGFR are evident when the analysis is stratified by ANCA type or by new diagnosis versus relapsing disease in patients with major renal involvement.15 For that reason, new clinical trials in well-defined homogeneous patient populations, selected according to their ANCA specificity and renal function, are needed. Until then, CYC should remain the first-line treatment in the induction of remission for patients with severe renal involvement. Similarly, further data are needed to unambiguously define the use of PLEX in the treatment of AAV with severe renal involvement. The results of the PEXIVAS study48 are expected to elucidate this question. Other general issues in the induction treatment of AAV vasculitis, including the use of oral or intravenous CYC, the preferred induction protocol for RTX, and the tapering of CCS during induction, remain to be elucidated, but a detailed discussion on these issues was beyond the scope of this review.
Finally, the continuous enrichment of knowledge on the pathogenetic mechanisms of this disease may be translated into new therapeutic strategies based on novel biological drugs; soon there may be therapeutic regimens with low doses of CCS or without CCS at all. Avacopan, a selective C5a receptor inhibitor, has proven to be an excellent glucocorticoid-sparing agent as showed in Phase II clinical trials.51,53 However, these data now require confirmation by an ongoing Phase III trial.52 Other agents are still under examination in promising preclinical studies.








