Abstract
Diarrhoea is a common complication after renal transplant and has a significant impact on quality of life, graft function, and mortality. The main causes of post-transplant diarrhoea are infectious and pharmacological. Mycophenolate mofetil (MMF) is an immunosuppressive medication widely used in kidney transplantation patients. Gastrointestinal side effects of MMF, such as nausea, vomiting, diarrhoea, and abdominal pain, mostly occur during the first months of treatment; however, late-onset diarrhoea does not exclude the diagnosis of MMF-induced colitis. MMF-induced colitis is associated with a wide histological spectrum, including inflammatory bowel disease-like, graft versus host disease-like, and ischaemia-like changes, which may lead to misdiagnosis. The complexity and severity of histological features might explain the variation in treatment response. Given the differences in the therapeutic management and prognosis of these histological changes, it is crucial to consider the diagnosis of MMF-induced colitis. The aim of this paper is to report a rare case of late-onset MMF-induced colitis with graft versus host disease-like features in a renal transplant patient who did not respond to MMF therapy withdrawal, and provide a review of data on this rare complication of immunosuppression.
INTRODUCTION
Diarrhoea is a common complication after renal transplant, particularly within the first year,1 and, although frequently overlooked, it has a significant impact on quality of life, graft function, and mortality.1,2 The main causes of post-transplant diarrhoea are infectious and pharmacological, commonly due to immunosuppressive medications.3 Mycophenolate mofetil (MMF) is an immunosuppressive treatment widely used in kidney transplantation patients due to its superiority in preventing rejection compared with azathioprine.4 Both MMF and enteric-coated mycophenolate sodium have been associated with post-transplant diarrhoea and other gastrointestinal (GI) side effects, such as nausea, vomiting, and abdominal pain, mostly occurring during the first 6 months of treatment.5 The incidence of GI toxicity has been reported to be between 40% and 85%, and it is the most common cause of drug withdrawal, which can lead to graft rejection.6
The use of MMF has been associated with many histological patterns, such as Crohn’s disease-like changes in the colon, erosive or ischaemic enterocolitis, and graft versus host disease (GVHD)-like colonic changes, which can make the diagnosis difficult.7,8 The aim of this paper is to report a case of late-onset MMF-induced colitis with GVHD-like features in a renal transplant patient and provide a review of data on this rare complication of immunosuppression.
CASE REPORT
The patient was a 50-year-old Caucasian male who had end-stage renal disease due to diabetic nephropathy. He required peritoneal dialysis for 4 years and underwent kidney transplantation in 2009 from a deceased donor with three major histocompatibility mismatches. The induction therapy involved basiliximab, tacrolimus, MMF, and prednisolone. In the first 3 months, the patient had an acute cellular rejection (Banff 1A) and was treated with pulse steroids; he then had a renal artery stent inserted during the postoperative period due to renal artery stenosis. His creatinine concentration on discharge measured 1.6 mg/dL and remained at this level throughout the follow-up period. Maintenance therapy was with tacrolimus, MMF, and prednisolone.
Nine years after the transplant, the patient developed a history of watery diarrhoea, general weakness, and anorexia, and lost 9 kg over 3 weeks. He had no fever or skin rash and there were no reported recent infections and no history of prior blood transfusions. The patient was medicated with tacrolimus, MMF, prednisolone, carvedilol, amlodipine, furosemide, calcitriol, and insulin. The dose of immunosuppression was stable for many years.
The patient was dehydrated, with a blood pressure of 98/54 mmHg and a body temperature of 36.1°C. He was anaemic (haemoglobin: 8.9 g/dL), with a normal white blood cell count and negative C-reactive protein. His kidney function deteriorated (creatinine: 3.1 mg/dL, urea: 118 mg/dL) and had hypokalaemia and metabolic acidosis. The MMF dose was 500 mg twice a day and his serum tacrolimus trough level was 6.8 ng/dL. He underwent fluid resuscitation and electrolyte reposition. The investigation for watery diarrhoea revealed negative Clostridium difficile toxin, negative stool cultures, negative cytomegalovirus antigenemia assay, and negative serologies for adenovirus and Epstein–Barr virus. Serum liver function tests were normal and a computed tomography (CT) scan of his head, chest, abdomen, and pelvis was unremarkable.
The patient’s diarrhoea persisted despite MMF withdrawal and there was a clinical deterioration with severe prostration and hypotension. He developed pancytopenia with a haemoglobin count of 6.8 g/dL, white blood cell count of 1.23×109 cell/L, and platelet count of 63×109 cell/L. His renal function aggravated due to hypovolaemia, with a maximum creatinine of 3.8 mg/dL and urea of 250 mg/dL; furthermore, he had severe hypoalbuminaemia (albumin: 1.9 mg/dL) as a result of malnutrition. He maintained the need for intravenous electrolyte and albumin reposition. A kidney biopsy was not performed due to the clinical deterioration of the patient. A colonoscopy examination was negative for viral infections and demonstrated mucosal erythema and extensive haemorrhagic spots in the ascending colon. Colon biopsies revealed lymphocytic infiltration of the epithelium, multiple eosinophils, sloughing and apoptosis of epithelial cells, and multiple crypts with apoptotic cells, consistent with GVHD (Figure 1).
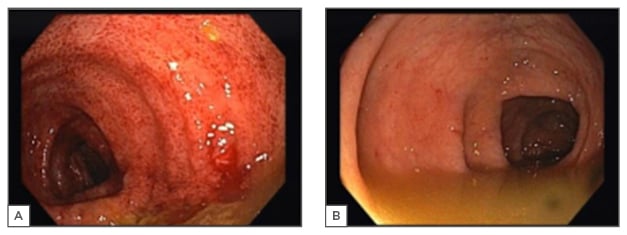
Figure 1: Colonoscopy findings in the patient.
A) The ascending colon with erythematous mucosa with multiple petechial lesions; B) The descending colon with normal mucosa.
The patient was started on parenteral nutrition support and bowel rest, and was treated with intravenous methylprednisone 1 g/day for 3 days, followed by 500 mg for 3 days, and then prednisolone 2 mg/kg/day for 7 days, which was then tapered slowly. After 4 days of steroid pulses the diarrhoea ceased, and by Day 10 of steroid therapy the patient was restarted on oral alimentation. Human leukocyte antigen (HLA) typing was performed in a peripheral blood sample but did not reveal the presence of donor T lymphocytes in the recipient’s blood, excluding GVHD in this patient.
This patient had two infectious complications related to his severe state of immunosuppression; namely, a Staphylococcus aureus methicillin-sensitive bacteraemia, which was treated with 3 weeks of flucloxacillin, and a nosocomial pyelonephritis to Klebsiella pneumoniae, which was treated with 3 weeks of cefuroxime. He was discharged after 2 months without diarrhoea and regained 50% of his lost body weight. He was maintained on tacrolimus and prednisolone only, with a kidney function that stabilised with a creatinine measure of 2.9 mg/dL.
DISCUSSION
MMF is widely used for the prevention of acute rejection following kidney transplantation.9 The drug is absorbed and hydrolysed to its active metabolite, mycophenolic acid (MPA), which acts by inhibiting inosine monophosphate dehydrogenase in the de novo pathway of purine synthesis, thus effectively suppressing lymphocyte proliferation, which is dependent on this pathway.10
MMF has a low risk of causing nephrotoxicity, cardiotoxicity, and diabetes10,11 but has the potential to affect both the upper and lower GI tract due to local and systemic disturbances.11 The exact mechanism of GI toxicity is unclear since enterocytes are only partially dependent on the de novo pathway of purine synthesis for proliferation. It has been demonstrated that the acyl glucuronide metabolite of MPA may play a role in inflammation by stimulating the release of interleukin-6 and tumour necrosis factor-alpha, and through the formation of neoantigens and subsequent activation of the immune system, causing either a hypersensitivity reaction or an autoimmune response. Also, the antibacterial effect of MPA can cause changes in the GI tract flora, promoting anaerobic growth and tissue damage.12-15
Although most cases occur within the first 6 months of therapy, a long latency period does not exclude the possibility of MMF-induced diarrhoea.16 Indeed, there have been no studies focussing on the chronological relationship between duration of MMF therapy and symptom onset.16 There have been sporadic reports of late-onset MMF-induced diarrhoea, with the latest in a heart transplant patient who presented after 13 years of stable MMF therapy.17,18
MMF-related GI mucosal injury may present with variable injury patterns, with the most common being a normal appearing mucosa.19 In a study by Selbst et al.,7 the histological changes in patients receiving MMF were categorised as normal or near normal (31%), inflammatory bowel disease-like (28%), GVHD-like (19%), ischaemia-like (3%), and self-limited colitis-like (16%). Calmet et al.8 also demonstrated histological variability in MMF-induced colitis and reported a GVHD-like pattern incidence of 8.3%. Liapis et al.20 further substantiated the wide spectrum of histological changes associated with MMF in 43 colon biopsies, with an incidence of 83% of inflammatory bowel disease-like changes and 18% of GVHD-like changes.20 The study also documented variable timing of diarrhoea onset and showed the main histopathological features of MMF-induced colitis were apoptosis and crypt distortion: characteristics similar to GVHD and inflammatory bowel disease (Table 1).20
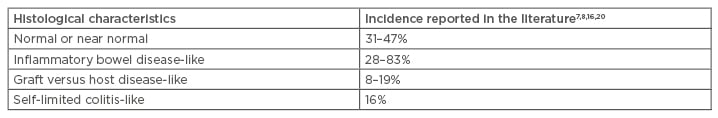
Table 1: Mycophenolate mofetil-induced colitis morphological spectrum.
In 2003, Papadimitriou et al.21 demonstrated similarities in the histological features of colitis secondary to MMF and GVHD. Likewise, Al-Absi et al.22 described five patients who presented with variable onset and duration of MMF-induced diarrhoea with colonoscopy findings similar to GVHD. These findings were prominent crypt cell apoptosis, enterocyte cytologic atypia, increased neuroendocrine cells, and glandular architectural distortion.21,22
The distinction between MMF and GVHD-induced colitis is clinically important since not only the aetiology and natural history but also the management of these disorders are different. In GVHD, the donor’s leukocytes dominate the recipient’s immune system and induce an immune reaction that causes a multisystem disorder.23 This is less common in solid organ transplantation than after haematopoietic stem cell transplantation. In kidney transplant patients, only six cases of GVHD have been described and these were associated with a high degree of HLA mismatch or donor HLA homozygosity; all cases were within the first year after transplantation, with a median time from transplant to GVHD of 111.2±90.4 days.24-29 The diagnosis of GVHD is made by a tissue biopsy documenting epithelial cell apoptosis and mononuclear cell inflammatory infiltrate, and evidence of the presence of donor lymphocytes by serological HLA typing, polymerase chain reaction-based microsatellite markers, or fluorescence in situ hybridisation analyses.23
The immune dysregulation caused by MMF induces proliferation of donor lymphocytes, which can generate a GVHD-like phenotype. Star et al.30 demonstrated that the presence and quantity of lamina propria eosinophils and endocrine cell aggregates, and the presence and degree of crypt distortion, apoptotic microabscesses, and hypereosinophilic crypts were features independently associated with MMF. Furthermore, the quantities of intraepithelial lymphocytes and endocrine cells were also significantly higher in patients with GVHD compared to those with MMF-induced colitis.30
The treatment for GVHD is based on increasing immunosuppression with corticosteroids and, depending on the severity of the symptoms, includes adding other immunosuppressive agents.21 In most cases of MMF-induced colitis, dose reduction or withdrawal is sufficient; however, if symptoms persist, steroid therapy may be required. There are controversial data on GI symptom improvement after conversion to enteric-coated mycophenolate sodium, which decreases local adverse effects but has similar systemic toxic effects to MMF.5,15,31,32 There is also one case report of MMF-induced ulcerative colitis with features of inflammatory bowel disease treated successfully with infliximab.33 The complexity and severity of histological features might explain the variation in treatment response.
The case detailed in this report was a challenging diagnosis of late-onset MMF-induced colitis that was unresponsive to MMF withdrawal and treated successfully with pulse steroids. MMF-induced colitis has rarely been reported with such a late presentation on a stable dose of immunosuppression, and cases of GVHD after kidney transplant have only been described within the first year. The patient had been receiving MMF therapy for 9 years and had tolerated it well up to symptom occurrence. Additionally, the biopsy findings of numerous eosinophils suggested a GVHD-like pattern of MMF-induced colitis, since GVHD was excluded by HLA typing. Although the diarrhoea persisted despite discontinuing MMF and a response was only possible with pulse steroids and high-dose prednisone, which is atypical for MMF-induced colitis, it was believed that the severity of the mucosal injury (similar to that seen in GVHD) was the cause of unresponsiveness to MMF withdrawal and the need for high-dose steroid therapy.
CONCLUSION
Diarrhoea associated with immunosuppression is a common complication after renal transplantation and has a significant impact on graft function and mortality. Although this adverse event commonly develops within the first year after kidney transplant, late-onset MMF-induced colitis must be considered as a differential diagnosis.
The wide morphological spectrum reported in MMF-induced colitis includes normal mucosa, inflammatory bowel disease-like, ischaemia-like, self-limited colitis-like, and GVHD-like features, which can result in misdiagnosis and a delay in the time to intervention. Given the differences in the therapeutic management and prognosis of these diseases, it is crucial to inform the pathologist of the clinical history of MMF therapy and to consider this diagnosis regardless of the duration of therapy. Treatment is complex and variable, ranging from MMF withdrawal to successful reports of specific immunosuppressive agents used to treat the histological pattern mimicked by MMF-induced colitis.








