Abstract
Prolonged intermittent renal replacement therapy (PIRRT) is an emerging form of renal replacement therapy in critically ill patients, but dosing data for antibiotics such as amoxicillin and cefepime are scarce and limited. This case report describes the effect of PIRRT on the plasma pharmacokinetics of amoxicillin and cefepime in a 69-year-old, critically ill patient with a polymicrobial intra-abdominal infection. Blood samples taken over 2 days, including a 7-hour PIRRT session, were analysed and a two-compartment model was used to describe cefepime and amoxicillin clearance and dosing requirements during PIRRT and off-PIRRT in this patient. Based on these data, an off-PIRRT dose of 1 g amoxicillin 12-hourly and cefepime 2 g daily with an on-PIRRT dose of 1 g amoxicillin 8-hourly and cefepime 2 g 12-hourly was deemed appropriate.
INTRODUCTION
Acute kidney injury and severe infections are common contributing factors to higher mortality in critically ill patients.1 For this patient population, continuous renal replacement therapy (CRRT) has traditionally been used as the form of renal replacement therapy (RRT). However, prolonged intermittent renal replacement therapy (PIRRT) is an emerging modality of RRT in the intensive care unit.2 PIRRT utilises conventional dialysis machines but runs over a longer time period with lower dialysate and blood flow rates which provides more stable haemodynamics and minimal solute disequilibrium compared to conventional intermittent haemodialysis.1 The advantages of PIRRT over CRRT include lower operating costs, decreased workload requirements and risk of infection because of the lack of bag handling, and increased patient mobility and participation in physical and occupational therapy.1,3,4 CRRT, however, has higher clearance rates of small and large solutes and the use of antimicrobials during CRRT has published references with dosing recommendations.1,4 Optimising antimicrobial dosing in critically ill patients is complex due to pathophysiological changes that can alter pharmacokinetics (PK) and pharmacodynamic properties of antimicrobials.1 Current antimicrobial PK data during PIRRT are largely limited to case reports or in silico dosing simulation studies. This is further complicated by the variability of PIRRT settings (e.g., blood flow, dialysate, ultrafiltration rates) and haemofilter characteristics, which all contribute to the challenge of antimicrobial therapy optimisation.2,5,6
The aim of this report is to describe the PK of amoxicillin and cefepime in a patient with polymicrobial intra-abdominal infection undergoing PIRRT and to provide dosing guidance in this setting.
MATERIALS AND METHODS
Patient Characteristics
A 69-year-old, 160 cm, 90 kg male presented with perforated sigmoid diverticulitis with faecal peritonitis. He underwent an emergency Hartmann’s procedure with formation of an end-colostomy. The patient had a complicated surgical admission; he underwent a further five laparotomies because of ongoing purulent/faecal collections which culminated into a further large bowel resection and stoma relocation. Initial intraoperative samples grew Escherichia coli, Streptococcus anginosus, Pseudomonas aeruginosa, Enterococcus faecalis, and mixed anaerobic bacteria. A further intraoperative sample taken 10 days later grew E. faecalis and P. aeruginosa which was reported to be resistant to piperacillin-tazobactam and meropenem. Wound dehiscence was noted after final surgical closure and swabs of the wound also cultured E. faecalis.
The patient sustained an acute kidney injury upon admission to hospital which temporarily required haemodialysis. Recovery of kidney function occurred 2 months into the patient’s hospital admission. During the PK sampling period, the patient was oliguric, with a urine output ranging between 0 and 20 mL/hour.
Antibiotic Dose and Administration
According to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) interpretive criteria for amoxicillin against E. faecalis and E.coli, clinical isolates are considered susceptible if the minimum inhibitory concentration (MIC) is ≤4 mg/L and ≤8 mg/L, respectively, and considered resistant when the MIC is >8 mg/L for both organisms.7 Amoxicillin against S. anginosus is inferred from benzylpenicillin, for which clinical isolates are considered susceptible if the MIC is ≤0.25 mg/L and resistant if the MIC is >0.25 mg/L.7 Lastly, for cefepime against P. aeruginosa and E. coli, clinical isolates are considered susceptible if the MIC is ≤0.001 mg/L and ≤1 mg/L, respectively, and resistant when the MIC is >8 mg/L and >4 mg/L, respectively.⁷
The patient was commenced on empirical intravenous (IV) antimicrobials for peritonitis on admission, followed by pathogen-directed therapy once intraoperative microbiology became available. During the PIRRT sampling period, the patient was prescribed IV amoxicillin 2 g over 30 minutes every 8 hours and IV cefepime 2 g over 30 minutes every 12 hours. Metronidazole 500 mg given intravenously every 12 hours was also administered in addition to this regimen.
Prolonged Intermittent Renal Replacement Therapy
PIRRT, using a Fresenius 5008 (Fresenius, Sydney, Australia), was conducted as a 7-hour treatment in the haemodiafiltration mode with a heparinised circuit and a 1.4m2 filter (Ultraflux® AV6700S, Fresenius), a blood flow rate of 200 mL/minute, dialysate flow rate of 240 mL/minute, and an approximate ultrafiltration rate of 233 mL/hour.
Blood Sampling
The patient had a total of eight samples taken over a 2-day period, which included one PIRRT session. Off-PIRRT, blood samples were taken at 0.0, 1.0, 3.5, and 6.0 hours post an amoxicillin dose, which also corresponded to a 6.0, 7.0, 9.5, and 12.0-hour (trough) post-cefepime dose. On-PIRRT, blood samples were taken at 0.0, 1.0, 3.0, and 6.0 hours post an amoxicillin dose, which also corresponded to a 3.0, 4.0, 6.0, and 9.0-hour post-cefepime dose. Samples were stored at -80˚C prior to assay at Pathology Queensland, Brisbane, Australia.
Drug Assay
Serum amoxicillin and cefepime concentrations were measured by validated high-performance liquid chromatography mass spectrometry at Pathology Queensland. Both analytes were linear from 0.1 to 2.5 mg/L, with an intra- and inter-run precision of <10%.
Pharmacokinetics Modelling
PK data were analysed by a nonparametric method with library package for R and for Pmetrics (Laboratory of Applied Pharmacokinetics, Los Angeles, California, USA) with testing of both one- and two-compartment models. An addition of a second clearance term was included to represent the dialytic clearance from PIRRT, which was tested and included. Both additive (λ) and multiplicative (γ) error models were tested for both drugs. Inspection of the log-likelihood ratio and goodness-of-fit plot was used to select the final models.
Consent
Informed consent was obtained to collect blood samples and to report this case.
RESULTS
Pharmacokinetic Results
The concentrations of amoxicillin during the entire sampling period were at least 5–10-fold higher than the breakpoint MIC of E. coli (8 mg/L) and E. faecalis (4 mg/L).⁷ Cefepime concentrations during the sampling were at least 29-fold higher than the breakpoint MIC of P. aeuroginosa (0.001 mg/L) and E. Coli (1 mg/L).⁷ A two-compartment model with clearance terms describing PIRRT clearance and non-PIRRT clearance adequately described the data.
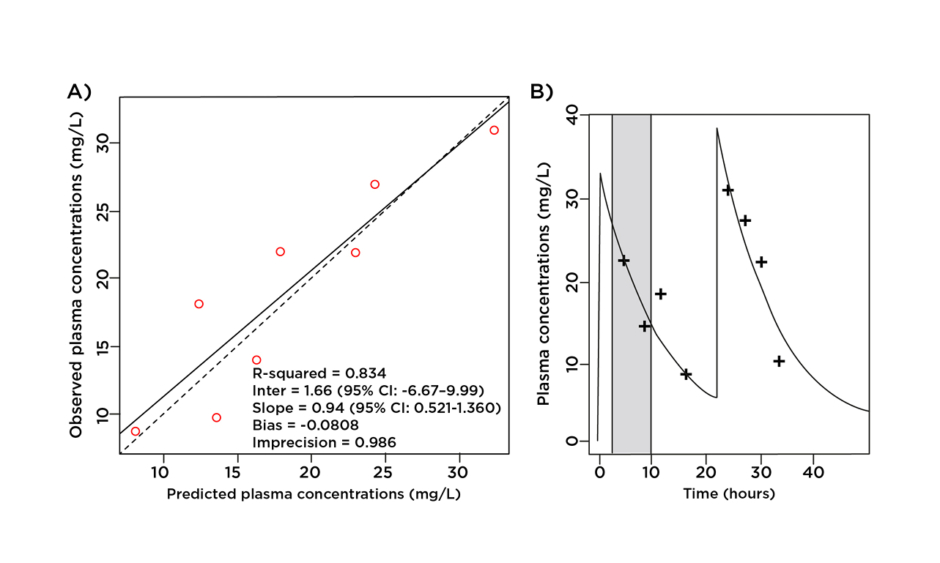
Table 1 describes the mean PK parameters of amoxicillin and cefepime. Figures 1 and 2 provide a graphical representation of the model goodness of fit of amoxicillin and cefepime, respectively, and confirm the adequacy of the model.
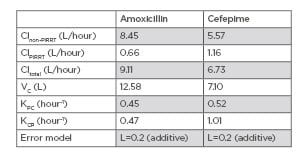
Table 1: Mean pharmacokinetic parameters of amoxicillin and cefepime in the patient.
Clnon-PIRRT: clearance of drug without prolonged intermittent renal replacement therapy; Clpirrt: clearance of drug with prolonged intermittent renal replacement therapy; Cltotal: clearance of drug during prolonged intermittent renal replacement therapy with native renal function; KCP: intercompartmental rate constant from central compartment to peripheral compartment; KPC: intercompartmental rate constant from peripheral compartment to central compartment; VC: volume of distribution of central compartment.
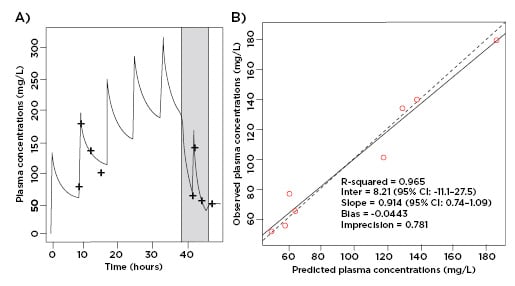
Figure 1: A) The predicted amoxicillin unbound plasma concentration time-profile versus observed data point, with PIRRT therapy shaded in grey. B) Observed-predicted plot for population and individual patient amoxicillin plasma concentrations.
CI: confidence interval; PIRRT: prolonged intermittent renal replacement therapy.
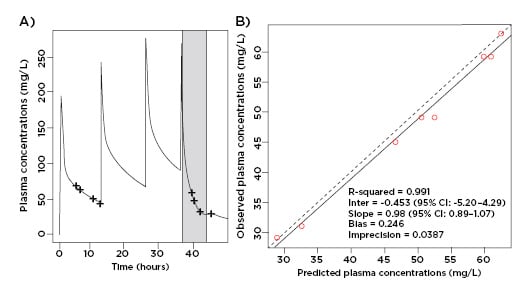
Figure 2: A) The predicted cefepime unbound plasma concentration time-profile versus observed data point, with PIRRT therapy shaded in grey. B) Observed-predicted plot for population and individual patient cefepime plasma concentrations.
CI: confidence interval; PIRRT: prolonged intermittent renal replacement therapy.
Clinical Outcomes
Around the sampling period, the patient exhibited signs of encephalopathy which treating clinicians suspected may have been because of cefepime toxicity. Because of this, ciprofloxacin was substituted for cefepime until antibiotics were subsequently ceased after a total of 6 weeks, with clinical, biochemical, and radiological resolution. The patient was successfully discharged from the intensive care unit after 38 days but remained in hospital to undergo medical treatment, followed by rehabilitation, for a further 6.5 months. He was discharged from hospital to transitional care to await placement in a rehabilitation facility for ongoing care.
DISCUSSION
To the authors’ knowledge, this is the first report describing the effect of PIRRT on amoxicillin PK. Amoxicillin is a β-lactam antibiotic which is predominantly renally cleared and has been documented to be removed by most forms of RRT. However, dosing recommendations for other RRT modalities cannot be readily transferred to patients undergoing PIRRT.8-13 β-lactams exhibit time-dependent pharmacodynamics, meaning the free drug concentration (∫) should be maintained over the causative organism’s MIC for the maximum amount of time (T>MIC) to maximise bacterial killing.1⁴ Experimental studies recommend a minimum 50%∫T>MIC should be targeted, although higher targets, as high as 100%∫T>4–5xMIC, have been suggested in critically ill patients with severe infections.4,15-17 In this patient, PIRRT significantly increased amoxicillin clearance compared with periods when PIRRT was not used (9.11 versus 0.66 L/hour). Because of the high amoxicillin clearance during PIRRT, inadequate amoxicillin dosing while a patient is on PIRRT could potentially lead to antimicrobial resistance or treatment failure.1⁸ However, in the sampling period the patient received an amoxicillin dose of 2 g every 8 hours on both PIRRT and non-PIRRT days, providing sufficient free drug levels to achieve 100%∫T>5xMIC. Given the low amoxicillin clearance when the patient was not on PIRRT and the high plasma levels achieved during the sampling period despite RRT, a dose of 1 g every 8 hours on PIRRT and 1 g every 12 hours on non-PIRRT days would be recommended.
Cefepime is a fourth-generation cephalosporin, with antimicrobial activity against Gram-positive and Gram-negative bacteria including P. aeruginosa, and is known to be removed by RRT.1⁹ Like other β-lactams, cefepime exhibits time-dependent bactericidal activity with studies suggesting similar targets ranging from 60–70%∫T>MIC to 100%∫T>4–5xMIC.1⁹,2⁰ There have been various dosing schedules suggested for patients receiving PIRRT, including a Monte Carlo simulation study which recommended a cefepime 2 g loading dose with 1 g every 6 hours while on PIRRT or cefepime 2 g at commencement of PIRRT and 3 g at the end of PIRRT.21 In this patient, PIRRT increased the cefepime clearance significantly (5.57 versus 1.16 L/hour). In spite of the higher clearance, a therapeutic target of 100%∫T5xMIC was easily achieved during the sample period. The use of cefepime in patients with renal impairment, in the intensive care unit, or of older age has been associated with an increased risk of neurotoxicity which can present as confusion, impaired consciousness, hallucinations, myoclonus, seizures, and encephalopathy.19-24 This neurotoxicity is attributed to an ability to cross the blood–brain barrier and exhibit concentration-dependent GABA antagonism.23,2⁴ Because this effect is concentration dependent, some studies suggest that a cefepime trough level of 36 mg/L is a highly accurate and sensitive threshold marker for cefepime-induced neurotoxicity, although this could be as low as 23 mg/L.23,24 Supratherapeutic cefepime concentrations could have contributed to the encephalopathy in this case, especially as no dosage adjustments were made when the patient was not being dialysed. A dose reduction to 2 g every 24 hours when not receiving PIRRT to reduce accumulation of cefepime is suggested.
The rate of diffusion, the principle mechanism of drug removal in PIRRT, is proportional to the surface area of the dialyser used. In this study, a dialyser with a surface area of 1.4 m2 was used. Using larger or smaller dialysers would result in proportionally different drug clearances.1
CONCLUSION
In conclusion, amoxicillin 2 g every 8 hours and cefepime 2 g every 12 hours both on PIRRT and off PIRRT resulted in concentrations well in excess of the MIC but at levels that could potentially be toxic in this patient. The authors recommend giving 1 g of the amoxicillin dose every 12 hours and 2 g of the cefepime dose every 24 hours during non-PIRRT periods because of the lower clearance when not on PIRRT. Given individual patient and PIRRT variabilities, however, further data are required to provide dosing recommendations for extrapolation to the rest of the patient population. The impact of PIRRT on metronidazole clearance was not available to be described in this paper.