Chairpeople: Mario Cozzolino
Renal and Dialysis Unit, ASST Santi Paolo e Carlo and
Department of Health Sciences, University of Milan, Italy
Speakers: Peter Stenvinkel,1 Jernej Pajek,2 Jarrin D. Penny3,4
1. Department of Renal Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
2. University Medical Centre, Ljubljana, Slovenia
3. Western University, London, Ontario, Canada
4. Kidney Clinical Research Unit, Lawson Health Research Institute, London Health Sciences Centre, London, Ontario, Canada
Disclosure: Cozzolino has participated in scientific advisory boards for Amgen, AstraZeneca, Baxter, GlaxoSmithKline, and Vifor Pharma, and has lectured at scientific meetings sponsored by Amgen, AstraZeneca, Baxter, Fresenius Medical Care, and Vifor Pharma. Stenvinkel has participated in scientific advisory boards for AstraZeneca, Baxter, Fresenius Medical Care, GlaxoSmithKline, Reata, and Vifor, and has lectured at scientific meetings sponsored by Astellas, AstraZeneca, Baxter, Fresenius Medical Care, Novo Nordisk, and Pfizer/Bristol-Myers Squibb. Pajek has received research grants and speaker fees from Baxter Healthcare. Penny has been a speaking consultant for Baxter, has received study sponsorship from Baxter Canada, and has received research funding from Baxter Healthcare, Kidney Foundation of Canada, London Health Sciences Centre, Satellite Healthcare, and the Western Graduate Research Scholarship.
Acknowledgements: Medical writing assistance was provided by Brigitte Scott, MarYas Editorial Services, Cowlinge, UK.
Support: The publication of this article was funded by Baxter. The views and opinions expressed are those of the speakers and not necessarily of Baxter.
Citation: EMJ Nephrol. 2022;10[1]:31-40. DOI/10.33590/emjnephrol/22C6991. https://doi.org/10.33590/emjnephrol/22C6991.
Meeting Summary
This symposium took place during the 59th Annual Congress of the European Renal Association (ERA), held in Paris in May 2022. Peter Stenvinkel, Department of Renal Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden, presented the pathological background of the association between the uremic milieu and inflammation and pathways for poor outcomes in patients on haemodialysis (HD). He described approaches to combat uremic inflammation, including interventions to decrease the production of inflammatory molecules and strategies to increase removal of inflammatory molecules by improved dialytic clearance using expanded haemodialysis (HDx) with a medium cut-off (MCO) membrane. Stenvinkel articulated that there are larger-middle molecular weight uremic molecules that are not removed by current dialysis strategies and that are associated with clinical outcomes, such as atherosclerotic cardiovascular (CV) disease, structural cardiac disease, and immunodeficiency. These molecules form a relevant group of uremic toxins to potentially be removed using MCO membranes. Stenvinkel also emphasised that dialyser loss is not the main factor for low serum albumin, and the latter is a predictor of poor outcome only in cases of high C-reactive protein (CRP). Finally, hypoalbuminemia predisposes to higher morbidity and mortality only in the presence of inflammation. Jernej Pajek, University Medical Centre, Ljubljana, Slovenia, explained that the predominant single causes of hospitalisation in patients with end-stage renal disease (ESRD) are CV causes and infections, and he explored the scientific plausibility for mechanisms linking CV disease, infections, and hospitalisation in patients on dialysis. He discussed studies evaluating intermediary mechanisms, indicating that dialysis with an MCO membrane may lead to lower CV morbidity and mortality, and described hard endpoint data that showed reduced hospitalisation rates with HDx compared with haemodialysis (HD). Jarrin D. Penny, Western University, London, Ontario, Canada, and Kidney Clinical Research Unit, Lawson Health Research Institute, London Health Sciences Centre, London, Ontario, Canada, discussed the importance of patient-reported outcome measures (PROM) in the management of patients on dialysis. She demonstrated the results from a dynamic PROM tool, demonstrating improvements in several parameters after patients were switched from high-flux HD to HD with an MCO membrane. Penny shared compelling testimonials alongside the changes in PROMs, in which patients described improvements in overall quality of life (QoL), general wellbeing, energy, sleep quality, appetite, pruritus, and restless legs with HDx compared with HD. She also discussed a patient case in which extensive itch and associated poor sleep, anxiety, and reduced QoL were greatly improved by 12 weeks of HDx. A patient described his experience of symptom improvement with HDx, symptoms returning on switching to HD, and again improving on reinitiation of HDx.Uremic Toxins and Inflammation
Peter Stenvinkel
Stenvinkel explained that the survival of patients on HD is significantly lower compared with the general population.1 There is regional variation in the risk of death in these patients, with significantly lower risk in China2 and Japan,3,4 two countries with a documented lower prevalence of inflammation, compared with the USA5,6 and Europe.7
Patients with chronic kidney disease (CKD) undergo premature ageing, including muscle wasting, osteoporosis, vascular calcification, and CV hypertrophy.8 According to Stenvinkel, inflammation has a role in this ageing process as it inhibits anti-ageing mechanisms and anabolic pathways, stimulates catabolic pathways, and, in concert with sympathicovagal imbalance and epigenetic changes with DNA damage, drives the premature ageing phenotype. Stenvinkel underscored how closely related inflammation is to telomere attrition, a main driver of premature ageing processes.9
Stenvinkel outlined that the causes of inflammation include exogenous factors such as central venous catheters, dialysis treatment, and gut dysbiosis; cellular factors such as cellular senescence and oxidative stress; tissue factors, such as sodium and fluid overload, and tissue hypoxia; and a plethora of uremic toxins such as calcium phosphate and indoxyl sulfate, which are major drivers of uremic inflammation.
Approaches to combat uremic inflammation include interventions aimed to decrease the production of inflammatory molecules. Stenvinkel highlighted lifestyle interventions, such as physical exercise and smoking cessation, and expressed a particular interest in the use of food as a medicine to reduce inflammatory markers (e.g., a balanced diet with low inflammatory potential) by bioactive nutrients. He also noted that some commonly used drugs have anti-inflammatory potential (e.g., statins, antidepressants, angiotensin-converting enzyme [ACE] inhibitors). Other strategies aim to increase the removal of inflammatory molecules, such as the use of targeted anti-inflammatory drugs, including N-acetylcysteine, bardoxolone, and anti-cytokine agents, and improved dialytic clearance using online haemodiafiltration (OL-HDF) and HDx.
Stenvinkel specified that uremic toxins affect virtually all organs in the body, contributing to issues such as kidney and CV damage, insulin resistance, infection, inflammation, intestinal dysbiosis, and cognitive impairment, which leads to reduced QoL and increased mortality.10
Uremic toxins found to have the highest toxicity evidence score include IK-6, TNF, and kynurenines.11 Stenvinkel explained that the current uremic toxins classification, however, does not help clinicians prescribe a dialysis regimen for patients with restless legs, nausea, cramps, fatigue, sexual dysfunction, pruritus, or prolonged recovery time after a dialysis session.10 He also emphasised that uremic solutes that are relatively large compared with urea (i.e., middle molecules such as p-Cresol sulfate and β2-microglobulin) are poorly cleared in conventional thrice-weekly HD, even with high-flux dialysers.12 Stenvinkel also pointed out that there are larger middle molecules that are not currently removed by dialysis strategies. These include fibroblast growth factor (FGF) 23, a hormone that helps to mitigate hyperphosphatemia in patients with kidney disease,13 and chitinase-3-like protein 1 (YKL-40), a protein secreted by macrophages, neutrophils, chondrocytes, endothelial cells, vascular smooth muscle cells (VSMC), and cancer cells.14 FGF 23 and YKL-40 are two independent predictors of poor outcome in patients with renal disease who are undergoing dialysis.13,14
Endogenous molecules that are dependent on kidney clearance are water soluble (<80% protein-bound) and are categorised by size, with biomarkers of known toxicity as shown in Table 1.10
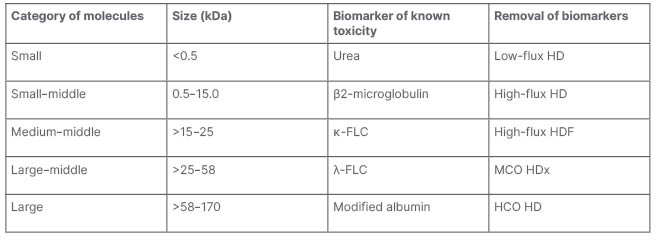
Table 1: Endogenous molecules dependent on kidney clearance.10
FLC: free light chain; HCO: high cut-off; HD: haemodialysis; HDF: haemodiafiltration; HDx: expanded haemodialysis; MCO: medium cut-off.
Stenvinkel proposed that studies are needed to test how changes in these biomarkers impact QoL. Exogenous (gut-derived) uremic toxins that are dependent on kidney clearance are small (<0.5 kDa) and ≥80% protein-bound molecules, such as homocysteine, p-Cresol sulfate, and kynurenines.10
The medium and large middle molecules are of clinical relevance as they have a role in atherosclerotic CV disease, structural cardiac disease, immunodeficiency,15 protein energy wasting,16 and systemic inflammation.17-19 Stenvinkel articulated that there are several larger middle molecules that are not removed by current dialysis methods, and that these form a relevant group of uremic toxins to potentially be removed using MCO membranes.20
In a randomised controlled trial of 172 patients, the reduction ratio for removal of λ-free light chain (FLC), κ-FLC, β2-microglobulin, complement factor D, and TNF was statistically significantly superior (p<0.001) for the MCO dialyser, Theranova 400 (Baxter Healthcare, Illinois, USA) compared with the high-flux dialyser, Elisio-17H (Nipro Group, Osaka, Japan).21 Stenvinkel summarised that HDx with the MCO dialyser was safe and efficacious, providing superior removal of larger middle molecules, including several putative uremic toxins, compared with a similar size high-flux dialyser.21
Stenvinkel also emphasised that hypoalbuminemia per se is not an independent predictor of increased mortality in dialysis patients, but in combination with inflammation it is a sign of poor prognosis.22 He noted that there is considerable albumin loss of 9–23 g associated with 4 hours of conventional HD with high cut-off membranes23 compared with only 2–4 g with MCO membranes.24 Stenvinkel observed that, on a weekly basis, albumin loss with MCO membranes is of similar magnitude to that in the urine of patients with macroalbuminuric Type I diabetes (1.3 g/day).25 In the presentation, studies26-30 were identified that evaluated the impact of albumin loss on serum albumin levels, and Stenvinkel noted that there was an association between albumin loss and decreased serum albumin in only four26,28-30 of the seven patient populations studied.22
There is considerable interest in the predictive impact of serum albumin. A study of 822 patients grouped according to albumin and CRP levels showed that after correcting for all possible confounding factors (such as age, sex, blood pressure, diabetes, smoking, subjective global assessment of nutritional status, and renal function), low serum albumin was a risk predictor only when there was concomitant high CRP.31 Stenvinkel concluded that inflammatory status should always be taken into account when using serum albumin for risk assessment in patients with CKD.31
Stenvinkel summarised the findings from HD and peritoneal dialysis (PD) studies. He explained that HD studies have shown that dialysis treatment modalities associated with improved outcomes have larger membrane surface and pore size, higher convective volumes, and transmembrane pressure, and are also associated with increased albumin loss into the dialysate.32 However, PD studies have shown that the pronounced daily protein losses (5–7 g/day) with this dialysis treatment modality rarely affect serum albumin levels.27 Furthermore, baseline peritoneal albumin and protein clearances are associated with signs of comorbidity, but do not have a measurable effect on patient survival.33
It can be speculated that albumin loss during dialysis may even be potentially beneficial because albumin is a major carrier for several small, protein-bound uremic toxins that are associated with endothelial dysfunction, and allowing some albumin loss into dialysate may increase their removal.34 In addition, irreversible post-translational modifications of albumin such as carbamylation, oxidation, and glycosylation are pronounced in the toxic uremic milieu; therefore, allowing some leakage into the dialysate and stimulation of functional production of unmodified hepatic albumin could be beneficial.34
Stenvinkel concluded that several middle molecule uremic solutes, particularly the larger middle molecules, promote systemic inflammation, and some are not removed by current dialysis strategies. Dialyser loss is not the main factor for low serum albumin in patients with CKD. Finally, hypoalbuminemia predisposes to higher morbidity and mortality only in the presence of inflammation.
Expanded Haemodialysis: Impact on Hospitalisations and Cardiovascular Events
Jernej Pajek
Pajek explained that the incidence of hospitalisation in the ESRD population is highest in patients on HD, slightly lower in those on PD, and much lower in transplant recipients.35 The predominant single causes of hospitalisation in patients with ESRD are CV causes and infections.35
Pajek referred to the association between the uremic milieu and inflammation and pathways for poorer outcomes in patients on dialysis described by Stenvinkel, and specified that middle and large-middle molecules, including IL-6 and TNF-α, are associated with inflammation, adhesion molecule expression, monocyte recruitment, macrophage activation, and vascular smooth muscle cell proliferation. All these events, Pajek noted, are associated with atherosclerosis, and may be a pathway to increased CV morbidity, hospitalisations, and mortality in patients on dialysis. Furthermore, larger middle molecular weight molecules, such as retinol-binding protein 4, FGF-23, and immunoglobulin light chains, are associated with one or more signs of secondary immunodeficiency (retinol-binding protein 4 is associated with impaired polymorphonuclear leukocyte [PMNL] function; FGF-23 is associated with impaired PMNL function and leukocyte inhibition; immunoglobin light chains are associated with impaired PMNL function and infectious mortality), and represent a potential pathway in which large-middle molecules may contribute to increased incidence of infections in ESRD.36 Pajek suggested that using MCO membranes may impact CV- and infection-related morbidity and mortality.
Pajek proposed that information on the potential impact of using MCO membranes on CV events in patients on dialysis can be gained through evaluation of intermediary outcomes, such as ACE expression, vascular calcification, and the presence of endothelial microparticles, also known as microvesicles, in the blood.
ACE expression was evaluated in a study in which blood from healthy volunteers was challenged with endotoxins, then their plasma was dialysed in vitro, and the effects of the plasma and dialysate on monocyte messenger RNA expression were evaluated.37 Plasma dialysed using an MCO or high cut-off membrane was associated with lower transcript expression of ACE messenger RNA and higher expression of the ACE2 isoform in monocytes compared with that using a high-flux membrane.37 As highlighted by Pajek, these results are relevant as a high ACE-to-ACE2 ratio is associated with an inflammatory and adhesive monocyte phenotype. The resultant dialysate using an MCO dialysis membrane caused greater induction of ACE compared with dialysate spent by dialysis with standard high-flux membrane. These results indicate that using an MCO membrane may impact on the inflammatory and adhesive phenotype of monocytes, and this may translate to reduced CV morbidity and mortality.37
The impact of HDx on vascular calcification was shown in a study in which serum from patients who underwent dialysis using an MCO membrane was associated with a 48% decrement in calcification of VSMCs in vitro compared with that from patients who underwent dialysis using a high-flux membrane.38 Lower calcium deposition in VSMCs was also observed using serum from patients who underwent HDx compared with regular HD in a small (20 patients), randomised, crossover study.39
Some uremic toxins, including p-Cresol sulfate and indoxyl sulfate, may activate endothelial cells to produce microvesicles, which are associated with vascular calcification, inflammation, oxidative stress, blood coagulation, and endothelial dysfunction.40 A study in which 63 patients on dialysis were randomised to continue with OL-HDF or switch to MCO dialysis membrane for 6 months showed a significant decrease in plasma endothelial microvesicles with MCO (p<0.05) compared with an increase with OL-HDF (p<0.05).41
According to Pajek, these three studies evaluating intermediary mechanisms indicate that dialysis with MCO may lead to lower CV morbidity and mortality; however, there are few hard endpoint data in this area.
A study conducted in South Korea with 80 patients randomised to HDx using the MCO membrane or OL-HDF for 1 year showed that there was no difference between the treatment groups for all-cause survival, CV survival, left ventricular ejection fraction, left ventricular mass index, and diastolic function of the heart.42 However, there was a slight increase in the calcium artery calcification score in the MCO membrane group.42
A 1 year observational study of almost 1,000 patients recruited in 2017 in Colombia compared quantitative data on mortality and hospitalisation rate with data from historical controls from the same HD network in 2014.43,44 There was numerically lower mortality in HDx patients compared with historical HD controls (8.54/100 patient years [PY] versus 14.6/100 PY) and lower rates of hospitalisation (0.79/PY versus 1.15/PY) and duration of hospitalisation (6.91 hour days/PY versus 9.6 hour days/PY).43,44
Similar data on hospitalisation were reported for a single-arm observational study in Colombia, in which 81 patients were switched from dialysis with a high-flux dialysis membrane to that with an MCO membrane.45 There was a notable decrease in the duration of hospitalisation with HDx compared with high-flux HD (4.41 versus 5.94 hospitalisation days per year; p<0.01).45
Further data on hospitalisations are available from the randomised controlled trial discussed by Stenvinkel in which 172 patients were randomised to either MCO dialyser or a high-flux dialyser.21 In this study, there was a 52% reduction in rate ratio of hospitalisation for the MCO dialyser compared with the high-flux dialyser.21,46 The aforementioned study of 20 patients who underwent HDx and regular HD in a randomised, crossover study39 also showed numerically higher hospitalisation rates in the HD group (p=0.079).47 This study also showed a lower infection incidence signal with HDx compared with regular HD (p=0.03),47 as did a meta-analysis of the impact of the MCO membrane compared with a regular high-flux membrane.46
Pajek concluded that CV disease and infections are the cause of the majority of hospitalisations in dialysis patients, and that in vitro studies indicate HDx may offer benefits beyond contemporary HD. A randomised controlled trial of HDx versus HD/HDF evaluating CV endpoints, as well as the rates and duration of hospitalisations, is still lacking. Future research should focus on an adequately powered randomised controlled trial. Finally, reimbursement issues need to be addressed.
Improving Patient-Reported Outcomes: The Impact of Expanded Haemodialysis Therapy on Quality of Life and Symptom Burden
Jarrin D. Penny
Penny began her presentation with a quote from a patient undergoing dialysis: “I understand why I need dialysis, but how can anything that makes me feel this bad not be hurting me?”
She explained that patients usually arrive for dialysis feeling generally well; however, after dialysis they feel drained and exhausted, and this provokes an important question: what are we doing to our patients? Penny recounted that many of the dialysis patients she has cared for over the last 25 years have told her that they feel their best just before they are about to undergo dialysis again, and she asked the audience to imagine living life knowing that the treatment that is keeping them alive is also making them feel unwell. Dialysis is associated with an erratic presentation of a range of symptoms that impact QoL, with no two days being the same for patients.
Penny referred to the science and compelling clinical indications associated with HDx therapy presented by Stenvinkel and Pajek, respectively, and questioned whether removing middle molecules using HDx therapy could potentially improve QoL and symptom burden in patients on dialysis. She outlined a study conducted to establish whether 12 weeks of HDx therapy had a direct impact on QoL and symptom burden measured by the London Evaluation of Illness (LEVIL) on a scale of 0 (poor) to 100 (excellent) for each of the six symptom domains (general wellbeing, energy, sleep, pain, appetite, breathing).48 The system automatically calculated an ‘overall QoL’ score, which was the average of all six domains combined.Penny described LEVIL as a dynamic, easy-to-use, timely PROM tool with a visual analogue scale that can be used repeatedly for real-time tracking of symptoms and patient response to treatment. She clarified that this tool is unlike traditional QoL assessment tools, which are cross-sectional, time-consuming, and do not represent the patient’s changing condition. A threshold LEVIL score of 70 was chosen by patients to distinguish between high (good) and low (bad) scoring, and this cut-off was used to stratify the data.
Penny described the study population as those who experienced low QoL and high symptom burden, tended to be on dialysis for longer, and had no residual renal output.48 The majority of the study population suffered from poor general wellbeing, and showed a significant improvement after 8 weeks of HDx therapy (p=0.001). All participants had poor energy levels at baseline, which also improved after 8 weeks of HDx therapy (p=0.001). Sleep quality improved after approximately 4 weeks of treatment, and continued to improve throughout the study (p=0.01 at 4 weeks and p<0.001 at 12 weeks). There was also an improvement in appetite and pain with HDx therapy; however, statistical significance was not reached. There was no change in breathing. When all the symptom domains were combined to give a measure of overall QoL, the results were consistent, showing an improvement after 8 weeks of HDx therapy (p<0.001). The LEVIL data for the initial 2-week observation period (conventional high-flux HD) followed by 12 weeks of HDx in the study are shown in Figure 1.48 This figure clearly shows the change in pattern of the LEVIL scores, reflecting an improvement in symptoms, after switching from high-flux HD to HDx.48 Penny suggested that the improved QoL and symptom burden after 8 weeks of HDx therapy in this study implied a putative clearance-based effect of symptom improvement. She noted that LEVIL can help clinicians determine which patients may benefit from HDx therapy, and to assess individual response to treatment over time, which is a useful resource management tool considering the financial constraints in dialysis therapy.
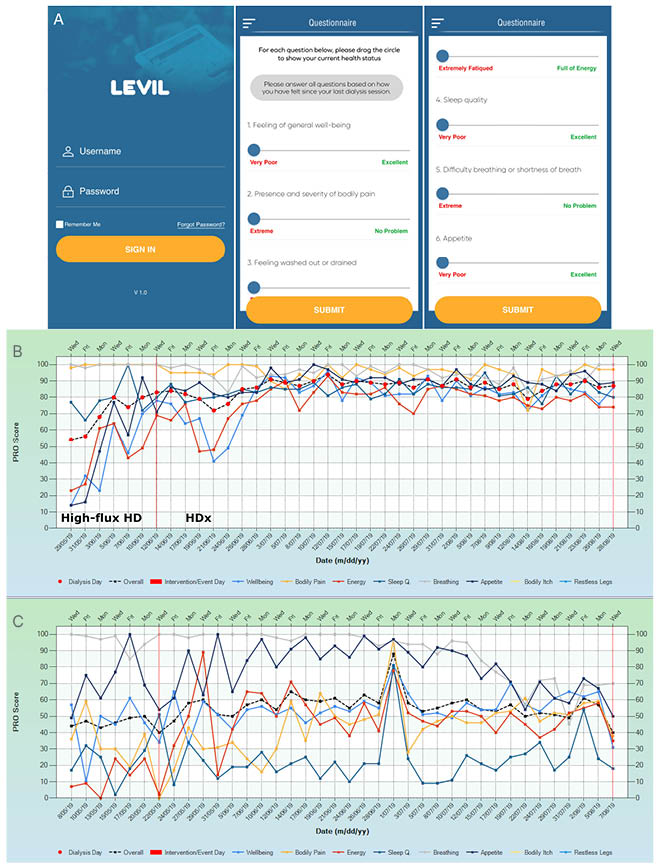
Figure 1: A) London Evaluation of Illness (LEVIL) application questions. B) Example of a patient’s response to haemodialysis as LEVIL graphical output. C) Variability in symptoms day-to-day using LEVIL, for conventional high-flux haemodialysis.
Penny summarised further information that was gleaned from discussions with the study patients, who described improvements in restless leg syndrome, post-dialysis recovery time, and pruritus on HDx therapy alongside their improvement in pre-existing LEVIL domains.
Penny then discussed an index case of a haemodialysis patient with debilitating pruritus and extreme levels of tissue sodium (measured by 23NaMRI). An 80-year-old male suffered from debilitating bodily itch, which meant that neither they nor their wife had slept for months; they also had raised vesicles on his shins, which added to their pruritus.49 Numerous medications and dermatology referrals were unsuccessful, and the patient was considering discontinuing dialysis because of their poor QoL. After 12 weeks of HDx therapy (in the study), however, the itch had resolved, the vesicles healed, and the patient’s (and their wife’s) sleep and QoL improved.49
In a separate case, the participant’s testimonial explained their improvement in appetite, sleep quality, and anxiety after starting HDx therapy. The patient explained the return of symptoms when they were switched back to high-flux HD, and admitted they were “really disappointed”. HDx was then reinitiated and, after a couple of days, appetite, sleep, and anxiety improved again. The patient emphasised that there was “definitely a difference” between the two treatments (HDx and high-flux HD), and Penny described this case as being extremely impactful.
Penny then outlined a 60-week multicentre trial extension in Canada and the USA, which aims to enhance the understanding of the symptoms that HDx impacts and incorporates these further learnings (restless leg syndrome, pruritus, mood, recovery time) with additional focus on the impact of HDx therapy at the microcirculatory level. She expects to provide insights on the trial, with preliminary results in 2023. Penny is also interested in the impact of HDx on cognitive function and sexual dysfunction.
Penny concluded with a question for the audience: if you could change just one thing about the therapy you deliver, what would it be?








