Meeting Summary
In many European countries, high-volume online haemodiafiltration (OL-HDF) is becoming the method of choice for treating patients with chronic kidney disease. The high convective (Qs >20 L/session) and diffusive properties of this treatment have been shown to be beneficial for patient survival. For optimum outcomes, the dialyser membrane must be able to cope with high transmembrane pressures. For this reason, the most widely-used membranes for this technique are synthetic and asymmetric in structure, making it easier for the membrane to divert the pressure away from its surface. However, patients allergic or sensitive to synthetic molecules, cannot access these high convective volumes (CV) reached in high-volume HDF, because alternative semi-natural membranes for allergic patients, such as cellulose acetate-based membranes, do not have adequate pressure-handling properties for high-volume HDF.
At this symposium, a new type of cellulose triacetate (CTA)-based membrane that is biocompatible, able to perform high-volume OL-HDF, and suitable for sensitive patients was introduced.
Anaphylactic Reactions to Haemodialysis Membranes
Professor Rafael Selgas
The hypersensitivity responses are categorised into two types, Type A and B. Type A response is a severe reaction that occurs within the first 30 minutes after the start of dialysis, it includes angioedema, dyspnoea, hypotension, cardiac arrest, and potentially death. This type of reaction requires immediate disconnection of the patient without returning the extracorporeal blood. The best-documented Type A reactions in dialysis are the reactions against ethylene oxide, hydrogen oxide (from dialyser reuse), formaldehyde, heparin, latex, or the combination of AN69 and angiotensin-converting enzyme inhibitors.1-4 The Type B response, also referred to as the unspecific response, is more common than the Type A response, but less severe. The symptoms include the aforementioned pulmonary leukostasis, leucopoenia, and hypoxaemia, and normally occur late on in dialysis, starting from around 15–30 minutes after the beginning of dialysis, or even later.5-7 These symptoms generally resolve during the session, and do not require patient disconnection.
Case Reports on Hypersensitivity Reactions in Dialysis
In 1988, it was reported that 4 out of 100,000 treatments lead to an anaphylactic reaction. In 2014, six case reports were published in which the patient had an allergic response against a synthetic dialyser membrane component.7 Patients were switched to a semi-natural CTA membrane and no longer experienced hypersensitivity reactions. It was suggested that the unknown factor activating these responses was one of the components present in the capillaries of the synthetic dialysis membrane, which was absent in the capillaries of the CTA membrane.7
To investigate the mechanisms involved in polysulfone (PS) hypersensitivity, basophil, T cell, and complement activation, were measured in acute-phase samples from two patients with anaphylactic reactions to PS-based membranes. Basophil and T cell activation, as well as higher serum tryptase levels, were detected in acute-phase samples compared with basal levels, suggesting the activation of mast cells and basophils. Complement levels (C3 and C4) were decreased in acute-phase samples from PS-allergic patients to a higher extent than in samples from control donors. In an ex vivo setting, basophils from PS-allergic patients exhibited increased degranulation, and T cells showed significantly increased activation after contact with PS-based membranes primed with low volumes of saline. No activation was detected in leukocytes from non-allergic patients under the same experimental conditions. Membrane priming with high volumes of saline abrogated activation of basophils and T cells; however, basophils from allergic donors showed significantly higher responses to FcɛR stimulation after contact with PS membranes. Basophil degranulation in allergic patients and serum tryptase levels during acute reactions support the activation of mast cells and basophils during hypersensitivity reactions to PS-based membranes.7
With these results in mind, this symposium discussed the implications of hypersensitivity reactions for patients undergoing haemodialysis (HD) and the possibility of keeping these patients on high convective therapies like high-volume OL-HDF.
Comparison of Old Versus New Generation Cellulose Triacetate Membrane in HD and HDF Treatment
Doctor Francisco Maduell
Several studies show that high-volume OL-HDF, when compared with HD, achieved better removal of uremic toxins, better control of hyperphosphataemia, anaemia, and nutrition; greater reductions in neurological symptoms and cardiovascular risk; as well as improved haemodynamic stability and survival.8-11 The ESHOL study, one of the most recent studies, was the first randomised controlled trial to demonstrate the superiority of OL-HDF over HD for all-cause mortality,9 a finding that was subsequently verified in other clinical trials. A pooled analysis of results from four studies involving a total of 2,700 patients found that OL-HDF versus HD reduced the risk of all-cause mortality and cardiovascular mortality by 14% and 23%, respectively.3 These benefits have further been demonstrated in several meta-analyses that reported significant risk reductions in all-cause and cardiovascular mortality with OL-HDF compared with HD.9,12,13 Further support for the reduced risk of mortality came from a French registry, which enrolled nearly 30,000 HD patients between 2008 and 2011. Of these patients, >5,000 participated in at least one HDF session and >2,000 were treated exclusively with HDF. All-cause mortality, cardiovascular mortality, and non-cardiovascular mortality were all reduced with OL-HDF compared with other forms of HD.14 A secondary analysis of three trials found that improved mortality rates were associated with a higher CV,9,15-17 suggesting that patients should receive a minimum of 21 L replacement volume and 23 L total CV. To achieve high-volume throughput and ultimately achieve higher survival rates, synthetic, high-flux membranes or cellulose triacetate membranes are required. All these results indicate that high-volume OL-HDF is more beneficial for patient outcomes.8-11
A literature review of the membranes capable of performing high-volume OL-HDF revealed several findings. In an experiment comparing the attributes of 11 dialyser membranes made of different materials, 11 patients received 11 dialysis sessions in which only the dialyser was changed.18 Observed blood flow rates were similar for all membranes, but transmembrane pressures were higher, and β2-microglobulin removal rates significantly lower for Tricea 190G (cellulose acetate) and BK-21P (polymethyl methacrylate [PMMA]) membranes. The investigators concluded that cellulose triacetate and PMMA membranes were of limited use for OL-HDF.18 A French study analysed 19 synthetic dialysers (not including PMMA) to assess their compatibility with the CVs recommended for postdilution OL-HDF.19 The investigators observed a direct relationship between CV, β2-microglobulin, and myoglobin reduction rates. When the amount of albumin lost in the dialysate was analysed, however, they found that 6 dialysers (Phylther HF 22SD [Bellco Srl, Mirandola, Italy], FDX-210GW, FDY-210GW [Nikkiso KK. Osaka, Japan], TS-2.1UL, (Toray Medical Company Ltd., Tokyo, Japan) FX 1000 HDF (Fresenius Medical Care, Bad Homburg vor der Höhe, Germany) and Rexeed-21A [Asahi Kasei Medical Co., Ltd., Tokyo, Japan]) were associated with albumin loss of >5 g per session, which they considered excessive. As a result, recommendations against the use of these 6 dialysers for OL-HDF were made. These studies show that, currently, the membrane chosen for high-volume OL-HDF is a synthetic membrane that is able to withstand high CV throughput but does not leach too much albumin.
The concerns with synthetic membranes are hypersensitivity reactions observed in some patients. Sànchez-Villeneuva et al.7 investigated six cases and found that hypersensitivity disappeared when synthetic membranes were replaced by CTA membranes. These findings were reviewed by Alvarez-de Lara and Martín-Malo,1 who reported the disappearance of hypersensitivity symptoms after a switch from synthetic membranes, which often contain polyvinylpyrrolidone, to CTA membranes. In their opinion, the fact that all clinics have seen at least a few hypersensitivity cases indicates that such reactions cannot be considered to be rare and should be monitored carefully. One of the current disadvantages of using a CTA membrane is that these membranes are less capable of performing high-volume OL-HDF. This is due to the uniform cross-sectional structure, which has a similar pore size on the blood-facing surface and the dialysate-facing surface. On the other hand, CTA membranes have a low electrical charge and minimal adsorption,20 excellent antithrombotic properties, and little residual blood loss after clinical HD. Furthermore, they have been associated with decreased triglyceride and increased HDL-cholesterol levels with long-term use, as well as decreased homocysteine and advanced glycation end products peptide levels.
These properties, in addition to the lack of hypersensitivity, make them excellent dialysers in many ways; however, it implies that patients on high-volume OL-HDF might have to return to HD or low-volume HDF treatments.
Up until now, there was an unmet need for a dialyser that combines the high-flux capability required for high-volume OL-HDF with reduced risk of hypersensitivity reactions. A new generation CTA membrane, the asymmetric triacetate (ATA) membrane, has been developed and has been proven suitable for high-volume OL-HDF. On the blood-facing side a smooth surface optimises pore distribution, whereas on the dialysate-facing side an asymmetric support layer, reduces pressure build-up in the membrane (Figure 1).21 The ATA membrane is similar to the CTA membrane in all characteristics except for a slightly increased wall thickness (25 µm versus 15 µm) and its asymmetric structure. An important advantage with this new membrane compared with the old CTA membrane is the reduction in transmembrane pressure. The ATA membrane has a lower baseline pressure in high-volume OL-HDF, which remains fairly constant throughout the dialysis session, while the pressure across the CTA membrane increases.21
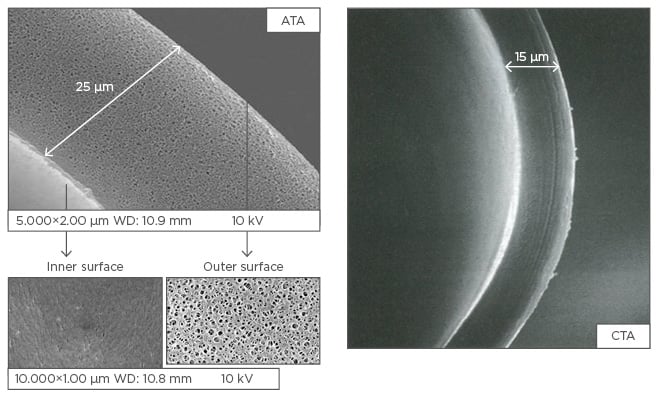
Figure 1: Asymmetric triacetate and cellulose triacetate membrane structure.
ATA: asymmetric triacetate; CTA: cellulose triacetate.
Adapted from Sunohara and Masuda.21
In a head-to-head comparison of the ATA and CTA membranes,22 the patient received two HD sessions with each of the two membranes, and two high-volume post-dilution OL-HDF sessions with each of the membranes. In all cases, dialysis time was approximately 5 hours; the mean blood flow was reported as 462 mL/min and dialysate flow as 500 mL/min. Performance was evaluated by measuring markers such as urea, creatinine, β2-microglubulin and myoglobin. Of particular interest to the investigators were the differences in albumin loss across the four dialysis sessions.
The study found no differences in initial, final and interdialytic weight, and no differences in initial and final haematocrit concentrations for high-volume OL-HDF (Table 1). The recommended minimum replacement volume (21 L/4 hours) was not achieved with the CTA but was comfortably achieved with the ATA membrane. Under HD conditions, the dialysate volume was similar for both membranes, but was significantly higher for the ATA membrane with high-volume OL-HDF (Figure 2A).
The results obtained for removal rates of larger molecules (β2-microglobulin [hl]Figure 2B[/hl], myoglobin [hl]Figure 2C[/hl], prolactin) were unexpected based on a previous study that reported increased CV with CTA membranes.23,24 The reduction rate of α1-microglobin was close to the cut-off point with the CTA membrane during HD, but nearly doubled during high-volume OL-HDF. Reduction rates of α1-acid glycoprotein were similar with both membranes in HD, while a significant reduction during OL-HDF was observed only with the ATA membrane. Albumin removal rates increased with OL-HDF compared with HD.10 Albumin loss was close to 1 g with ATA and >1 g with CTA in HD, and 1.7 g for both membranes in OL-HDF (Figure 2D).
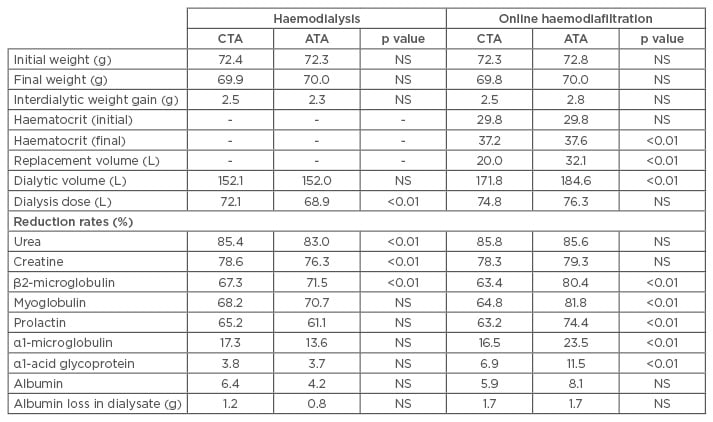
Table 1: Comparison of cellulose triacetate and asymmetric triacetate performance under haemodialysis and online haemodiafiltration conditions.8
ATA: asymmetric triacetate; CTA: cellulose triacetate; NS: not significant.
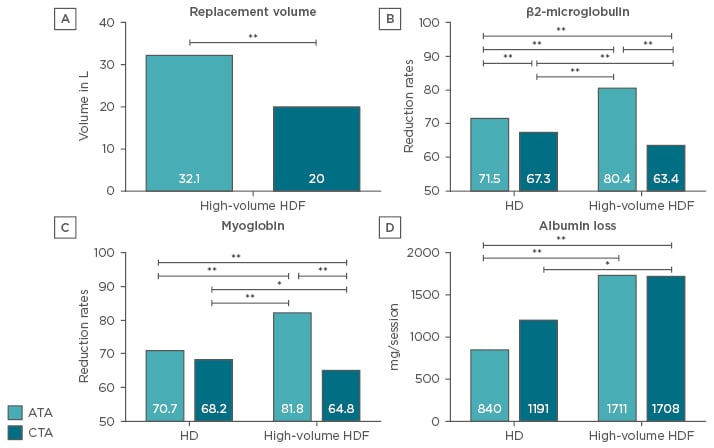
Figure 2: Performance comparison of the cellulose triacetate and asymmetric triacetate membrane.
Comparison of the replacement volume in high-volume OL-HDF between the ATA and the CTA.
A) reduction rates of β2-microglobulin; B) and myoglobin; C) and the loss of albumin; D) between the ATA and CTA membrane in HD and high-volume OL-HDF.
*p<0.05; **p<0.01.
ATA: asymmetric triacetate; CTA: cellulose triacetate; HD: haemodialysis; HDF: haemodiafiltration; OL-HDF: online haemodiafiltration.
Other studies comparing the ATA and CTA membranes are ongoing in Madrid, Spain (NCT03105817)25 and London, UK (NCT02546037).26 A recent study comparing the ATA membrane and the polynephron membrane, presented at the ERA-EDTA 2017 congress, found no differences in dialysis load, β2-microglobin, myoglobin, and albumin reduction rates in a small study population of six patients. Albumin loss was 1 g with a CV of 26 L.15
The newly developed ATA membrane is a suitable membrane for high convective therapies reaching a CV well above 20 L/session. Performance-wise, it demonstrated benefits across all markers analysed, including the middle molecular weight molecules, without losing large amounts of albumin, making it a good alternative for current synthetic high-volume HDF membranes.
Biocompatible Characteristics of a Newly Developed Smooth Surface Membrane
Doctor Tadashi Tomo
HD and other similar processes have many bioincompatible factors. These presumably induce inflammation, which can lead to the development of complications (e.g. atherosclerosis); hence the need to reduce micro-inflammation. Biocompatibility of the treatment is influenced by the materials and chemicals used in the treatment but also the type of treatment provided.
When we look at the treatments provided, data from the Japanese J-DOPPS III study indicate that increased baseline levels of the inflammatory molecule C-reactive protein (CRP) is associated with increased death risk, which implies that techniques that reduce CRP levels could reduce chronic inflammation and ultimately death.27 Several studies have demonstrated that dialysis with OL-HDF results in lower CRP levels and lower mortality rates than with HD, making the HDF technique an attractive technique that is able to reduce micro-inflammation.8,28,29 To understand some of the concerns around biocompatibility, differences between pre-dilution HDF and postdilution HDF were assessed.30 In pre-dilution HDF, some fluid is injected into the inlet of the haemodiafilter so that contact between the white blood cells, monocytes, and membrane is decreased, but linear velocity and shear stress increase. The opposite is true for post-dilution HDF, where linear velocity is decreased and contact between white blood cells, monocytes, and membrane are increased due to haemoconcentration.
Alongside the dialysis technique used, the dialyser membrane used has a major impact on biocompatibility of the treatment, because the body continues to recognise it as a foreign substance. In the long term, even small inflammatory reactions between the dialysis membrane and blood cannot be overlooked. An improvement in the general biocompatibility of membranes and a reduction in contact between membrane surface and blood cells are pivotal in preventing micro-inflammation.
The new ATA membranes are designed to perform well in both sections: achieve good clearances in high-volume HDF and have as little as possible interaction with blood cells to minimise microinflammation. Figure 3 shows the differences in the surface roughness of ATA, CTA, and PS membranes with the ATA membrane at the smooth and the PS membrane at the rough end of the spectrum. The build-ups of the membranes are also different. The CTA membrane is symmetrical, limiting its capabilities in high-volume treatments, and the ATA and PS membranes are asymmetrical, making them capable of withstanding higher convective therapies than the CTA membrane.
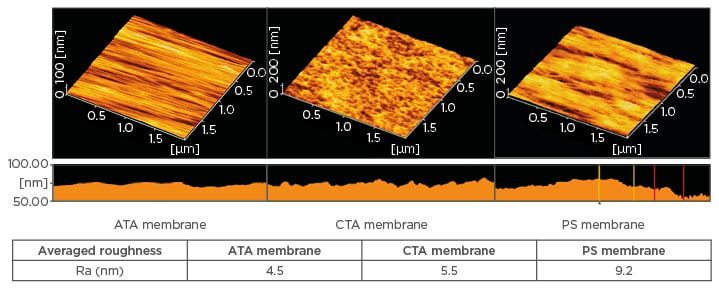
Figure 3: AFM analysis of the surface roughness of the asymmetric triacetate, cellulose triacetate, and polysulfone membranes.21
AFM: atomic-force microscopy; ATA: asymmetric triacetate; CTA: cellulose triacetate; PS: polysulfone.
To investigate the inflammatory properties of different membranes and treatment types, the ATA, CTA and PS membranes were investigated in an ex vivo study. Micro modules consisting of fifty fibres from three membranes (CTA membrane from FB-U [Nipro Corporation Osaka, Japan], ATA membrane from FIX-210Seco [Nipro Corporation, Osaka, Japan], and PS membrane from FX-180 [Nikkiso KK. Tokyo, Japan]), each 16 cm in length, were exposed to blood collected from healthy volunteers under HD, pre-dilution haemofiltration (HF)/HDF and post-dilution HF/HDF conditions. Blood flow rates, dialysis flow rates, and substitution-flow rates were adjusted to be equivalent with those of liner velocity used in clinical practice. After 15 mins of exposure, neutrophils were separated from the blood samples and neutrophil counts were adjusted to 1.5×106 /mL by dilution with phosphate buffered saline. After 10 µL luminol and 100 µL t-buOOH were added to 200 µL of this sample, the free-radical content was measured. The concentration of platelet-derived micro particles (PDMP) in the plasma was measured by the ELISA method.
Neutrophil-induced free radical formation is a good indicator for micro-inflammation. When tested in circulated blood that passed through the ex vivo circuit containing the different dialysis membranes and applying different treatment modalities, it was found that the ATA membrane in comparison with the CTA and PS membranes (tested under blood circulation without dialysate) had the lowest amount of free radical induction and PS had highest. In the HD condition, the amount of free radical induction from ATA and PS does not show a significant difference, and CTA had the highest. The variation in results between the two conditions was considered to be caused by dialysate or back filtration. In the pre-dilution HF and HDF, and the post-dilution HF and HDF conditions, ATA had the lowest amount of free radical induction and PS had highest. These results might be explained by the ATA membrane inducing less radical stress, usually caused by blood concentration or shear rate, under HF and HDF conditions.
The formation of PDMPs play an important role in the clotting process by stimulating platelets and monocytes leading to aggregation of platelets and potentially hypercoagulability, as well as promoting secretion of adhesion molecules. PDMP may act as a biomarker of the biocompatibility of dialysis membranes. When assessing this marker, PDMP levels were lower with the ATA membrane under HD, pre-dilution HDF and post-dilution HDF conditions, however, these results were not demonstrated during the blood flow assessments under pre-dilution HD and post-dilution HD conditions. Further investigations are required to assess the impact of PDMP levels on membrane compatibility in more depth as current assumptions are based on limited data.
In conclusion, poor biocompatibility of dialysis membranes is implicated in promoting micro-inflammation in the blood vessels of the patient. The newly developed ATA membrane appears to have an improved biocompatibility profile in high convective therapies compared with earlier membranes.








