Speakers: Loreto Gesualdo,1 Sian Griffin,2 Pierre-Louis Tharaux3
1. Nephrology, Dialysis and Transplantation Unit, Azienda Ospedaliero Universitaria Consorziale Policlinico, University of Bari, Italy
2. Department of Nephrology and Transplantation, University Hospital of Wales, Cardiff, UK
3. Institut National de la Santé et de la Recherche Médicale (Inserm), Université Paris Cité, Paris Cardiovascular Center (PARCC), France
Disclosure: Gesualdo has received research funding from Abionyx and Sanofi; speaker activity for Fresenius, Estor, Werfen, Astellas Pharma, AstraZeneca, and Travere Therapeutics; and consulting activity for Sandoz, Sanofi, Baxter, Mundipharma, Estor, Pharmadoctor, Travere Therapeutics, AstraZeneca, Gs Pharma, and Novartis. Griffin has disclosed consultancy for Hansa Biopharma, Alexion Pharmaceuticals, Vifor Pharma, AstraZeneca, and Travere Therapeutics. Tharaux has received consulting fees from Travere Therapeutics.
Acknowledgements: Writing assistance was provided by Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Disclaimer: The opinions expressed in this article are not necessarily those of Vifor Pharma.
Support: Publication of this feature was supported and reviewed by Vifor Pharma and reviewed by Travere Therapeutics.
Citation: EMJ Nephrol. 2022;10[1]:20-29. DOI/10.33590/emjnephrol/22C0912. https://doi.org/10.33590/emjnephrol/22C0912.
Meeting Summary
IgA nephropathy (IgAN) and focal segmental glomerulosclerosis (FSGS) are rare primary glomerulopathies, though the incidence of IgAN is greater. Endothelin 1 (ET-1) and angiotensin II (Ang II) are implicated in the development and progression of IgAN and FSGS. Both conditions impact health-related quality of life (HRQoL) and may lead to kidney failure. IgAN and FSGS are both evidenced clinically by proteinuria, with a greater degree of such associated with more progressive disease and shorter times to kidney failure. Accordingly, the reduction of proteinuria in patients with these conditions is a key target. Currently, IgAN and FSGS treatments are unsuccessful or only partially successful in a number of patients. Immunosuppressant therapy is first-line for primary FSGS and utilised for patients with IgAN who remain at high risk of progression despite maximal supportive care; however, while effective, there is a significant risk of toxicity and relapse is frequent. A number of clinical trials are ongoing to investigate the use of non-immunosuppressive agents in the management of these conditions. The dual endothelin Type A receptor/Ang II subtype 1 receptor (ETAR/AT1R) antagonist (DEARA) sparsentan is currently being assessed as a means to control kidney disease progression. Interim study results show that sparsentan can lead to greater reductions in proteinuria than AT1R antagonism alone in IgAN and more patients reaching partial remission (PR) in FSGS. Herein, a symposium by leading experts at the European Renal Association (ERA) 59th Congress in Paris, 19th−22nd May 2022, is presented. It highlights IgAN and FSGS and the role of proteinuria in these conditions, and how targeting ET-1 and Ang II can lead to a reduction in proteinuria in IgAN and potential FSGS PR.Introduction
Primary glomerulopathies include IgAN and FSGS, both of which are diagnosed following kidney biopsy.1 One clinical sign associated with higher progression rates of kidney disease, kidney failure, and death in these conditions is proteinuria.2,3 As such, the Kidney Disease: Improving Global Outcomes (KDIGO) 2021 Clinical Practice Guideline for the Management of Glomerular Diseases states that proteinuria has disease-specific relevance for prognosis and treatment decision-making in glomerular disease.1
Herein is a summary of a symposium delivered at the ERA 59th Congress, where Loreto Gesualdo, Nephrology, Dialysis and Transplantation Unit, Azienda Ospedaliero Universitaria Consorziale Policlinico, University of Bari, Italy, first discussed the clinical significance of proteinuria in IgAN therapy; and then Sian Griffin, Department of Nephrology and Transplantation, University Hospital of Wales, Cardiff, UK, evaluated proteinuria with respect to FSGS. Finally, Pierre-Louis Tharaux, Institut National de la Santé et de la Recherche Médicale (Inserm), Université Paris Cité, Paris Cardiovascular Center (PARCC), France, discussed the role of the peptide ET-1 and the hormone Ang II in both IgAN and FSGS, and described how targeting these pathways reduce proteinuria and could promote long-term kidney health.
Spotlight on IgA Nephropathy: Clinical Significance of Proteinuria
Loreto Gesualdo
Though rare, the immune-complex mediated glomerular disease IgAN is one of the most prevalent primary glomerulonephritis worldwide. Although peak incidence occurs in the third and fourth decades of life, IgAN can occur at any age. Incidence rates vary widely according to how IgAN is assessed, from between 0.03−4.5/100,000 persons/year in children and teenagers and 0.2−5.7/100,000 persons/year in the general population (including children).4,5 In one study, there was a 1.53-fold increased risk in all‐cause mortality and a 6-year reduction in life expectancy compared with age/sex matched controls.6 In another, 53% of patients with primary IgAN developed kidney disease or died within a median follow-up time of 9.2 years.7
IgAN can profoundly impact a person’s quality of life due to symptoms, such as pain and fatigue, and is associated with depression and anxiety,8 which are often related to uncertainty and complications with therapy.9 In patients who progress to kidney failure, HRQoL is even more affected, with substantial adverse effects on mental health and physical functioning.10-12
As shown in Figure 1,9,13,14 increased levels of galactose-deficient IgA1 (Gd-IgA1) in IgAN lead to increased production of Gd-IgA1 antibodies, both of which can increase mesangial cell deposition of Gd-IgA1-containing immune complexes. This results in mesangial cell activation and proliferation, which both stimulates and is stimulated by mediators including Ang II and ET-1. A pathological cycle can occur whereby downstream consequences of these processes, including inflammation, extracellular matrix production, podocyte cytoskeletal alterations, and endothelial dysfunction, may also lead to increased mesangial cell proliferation as well as Ang II and ET-1 production. The result is a compromised glomerular filtration barrier and subsequent proteinuria and haematuria, leading to tubulointerstitial inflammation, fibrosis, and, ultimately, progressive decline in kidney function.9,13,14
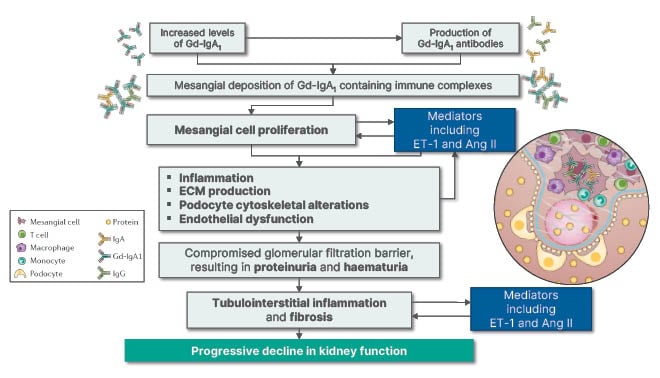
Figure 1: Decline in kidney function in IgA nephropathy. Ang II: angiotensin II; ECM: extracellular matrix; ET-1: endothelin 1; Gd-IgA1: galactose-deficient IgA1.
Gesualdo explained that because the estimated glomerular filtration rate (eGFR) decreases with increasing proteinuria, it is recommended that both factors be taken into consideration when assessing kidney function.1 The rate of decline in kidney function in IgAN is most strongly predicted by sustained proteinuria, with each incremental g/day >1 g being associated with a 10- to 25-fold more rapid rate of kidney function decline and similar differences in kidney survival.15
The goal of therapy in IgAN, in accordance with the KDIGO guidelines, is to slow the rate of progression to kidney disease through management of proteinuria to at least <1 g/day, and blood pressure to <120 mmHg systolic blood pressure. With this in mind, proteinuria has now been incorporated into a risk stratification protocol through the International IgAN Prediction Tool, as recommended by the KDIGO guidelines.1 This tool incorporates clinical and histologic data to provide a prognosis at the time of biopsy to help identify patients at high risk of rapid disease progression and requiring urgent care to protect kidney function.1,16,17
Magnitude and duration of proteinuria reduction have been shown to impact long-term clinical endpoints in IgAN. For instance, a retrospective, multi-ethnic cohort study found that each 3-month period of proteinuria remission (defined as ≥25% reduction in proteinuria from peak value after biopsy and an absolute reduction in proteinuria to >1 g/day) was associated with a 9% relative risk reduction in kidney failure or a 50% reduction in eGFR decline over a median follow-up of 3.9 years.18
In addition, an analysis of patient registry data predicted that a 30% reduction in proteinuria could lead to a 50% lower risk of kidney failure and an increase in median time to kidney failure from 12.4 to 23.1 years.19 Proteinuria has successfully been used in clinical studies as a surrogate endpoint for the effect of treatment on slowing progression to kidney failure, as evidenced by a meta-analysis of 13 controlled trials that showed an association between the effect of treatment on proteinuria and treatment effects on a composite of the time to the first occurrence of a doubling of serum creatinine level, kidney failure, or death.20
Gesualdo noted, however, that there remains a high unmet clinical need in IgAN therapy, with approximately half of all patients remaining at high risk of progression due to proteinuria levels of >0.75−1.00 g/day for ≥90 days1 despite 3 months of therapy.1,21,22 The KDIGO guidelines for the management of glomerular diseases indicate that patients with IgAN who remain at high risk of progressive chronic kidney disease despite maximal supportive care can be considered for a 6-month course of immunosuppressive therapy with corticosteroids. However, the risk/benefit profile of glucocorticoids must be individually discussed for those with eGFR ≥30 mL/min/1.732, and maximal supportive care should be considered for patients with eGFR 30 mL/min/1.732.1
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARB) are also recommended for the treatment of IgAN. However, condition-specific studies are limited, and data supporting their use are of low to moderate quality.1 There is also debate over whether renin-angiotensin system (RAS) blockade with ACEis/ARB alone is sufficient, or whether therapy should also include immunosuppression.23,24
In the TESTING study, over a mean 4.2-years follow-up, the hazard ratio for the primary endpoint (40% decline in eGFR, kidney failure, or death due to kidney disease) was 0.53 (95% confidence interval: 0.39−0.72; p<0.001) for those receiving methylprednisolone (n=136) compared with a placebo group (n=126). However, serious adverse events were more frequent with methylprednisolone (10.9%) versus placebo (2.8%), especially in those with full-dose therapy (maximum 48 mg/d), where figures were 16.2% and 3.2%, respectively.25 However, as corticosteroid therapy carries a significant risk of toxicity, this approach should be avoided in susceptible patients,1,24,26 while there is also an increased risk of infections in IgAN with the use of steroids and other immunosuppressants.24,26
Several Phase III trials are currently investigating novel therapeutic approaches to target proteinuria in IgAN. The DEARA sparsentan is being investigated in the PROTECT trial, in which the primary interim endpoint (PIE) is a change in urinary protein-to-creatinine ratio (UP/C) from baseline to Week 36. In this 114-week study, with an open-label extension period of up to 156 weeks, approximately 404 patients with persistent overt proteinuria who remain at high risk of disease progression despite therapy have been randomly assigned in a 1:1 ratio to either sparsentan or irbesartan (an ARB).27
Other studies include the delayed release of budesonide that is being investigated in the NEFIGARD trial, which has a PIE of change in UP/C from baseline to 9 months.28 This corticosteroid has recently received accelerated approval by the U.S. Food and Drugs Administration (FDA).29 Narsoplimab, a mannan-binding lectin serine protease 2, is being investigated in the ARTEMIS-IgAN trial, which has a PIE of change in 24-hour urine protein excretion from baseline to Week 36,30 while the APPLAUSE-IgAN trial of the factor B inhibitor (alternative complement pathway), iptacopan (LNP023), has a PIE of ratio of UP/C from baseline to 9 months.31 Finally, the ETAR antagonist atrasentan is being investigated in the ALIGN trial, with a PIE of change in UP/C ratio from baseline to Week 24.32 “After 25 years,” concluded Gesualdo, “finally we’re getting […] new drugs that may change the natural history of IgANs.”
Spotlight on Focal Segmental Glomerulosclerosis: Clinical Significance of Proteinuria
Sian Griffin
FSGS is uncommon, with a global incidence of 0.8/100,000/year depending on study and clinical assessment.5 Griffin noted that the condition has a significant impact on patients. An estimated 15% of glomerular disease diagnoses in Europe are due to FSGS,33 and it accounts for approximately 20% of paediatric and 40% of adult nephrotic syndrome cases, the larger figure of the latter being due to greater heterogeneity of causes.34,35 This is of concern as kidney failure occurs in up to 45% of cases over 10 years and is associated with an increase in mortality.36
Griffin described how the profound impact of nephrotic syndrome is seen on the lives of patients in her clinic, and can be especially devastating for children and young adults and during pregnancy. Studies have revealed that the impact includes physical symptoms, such as shortness of breath, severe oedema, and fatigue,37,38 as well as an increased risk of cardiovascular problems, such as acute coronary syndrome, heart failure, venous thromboembolism, and death.35 FSGS has also been shown to lead frequently to HRQoL issues such as anxiety, depression, poor sleep, and a reduced ability to socialise.37 Pregnancy carries a significant risk to patients with FSGS, as well as the fetus.1 As such, Griffin noted that patients with FSGS require counselling to understand the disease and its potential consequences.
As can be seen from Figure 2,14,39 the site of primary injury in FSGS is the glomerular podocyte. Such injury can arise from internal (genetic predisposition, unknown circulating factors, disrupted signalling pathways, and mechanical load) or external (toxins including anthracyclines, mechanistic target of rapamycin inhibitors, and anabolic steroids, and viral infections such as HIV and cytomegalovirus) factors.14,39 The podocyte, according to Griffin, is usually a very resilient cell, but injury can lead to detachment from the underlying membrane, cell death, and an impaired glomerular filtration barrier. There is a critical threshold of podocyte loss, but amplification and persistence of injury (via inflammatory mediators including ET-1 and Ang II) can lead to podocyte depletion as it is a terminally differentiated cell with little or no regeneration capacity. This results in large areas of bare basement membrane and connections formed with overlying parietal epithelial cells, which can become activated. There is also ongoing inflammation with obliteration of the capillary loop and segmental sclerosis. This can be seen histologically as progressive FSGS and manifests clinically as proteinuria.39
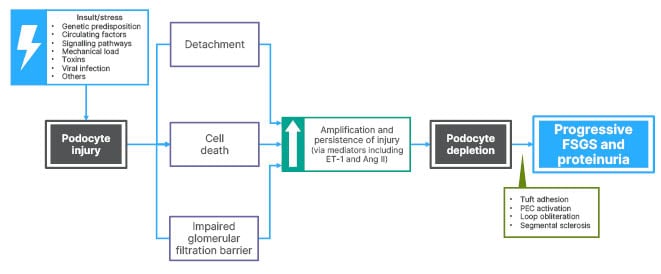
Figure 2: Podocyte injury and depletion in focal segmental glomerulosclerosis.
Ang II: angiotensin II; ET-1: endothelin 1; FSGS: focal segmental glomerulosclerosis; PEC: parietal epithelial cell.
In 2021, the KDIGO guidelines for the management of glomerular diseases were updated so that FSGS is classified based on proteinuria, aetiology, and histological presentation of a biopsy. Primary FSGS is the classical cause associated with circulating factors, as evidenced by successful treatment by plasmapheresis in rapid and recurrence of FSGS following kidney transplantation.1 This typically presents abruptly with FSGS lesions, extensive foot process effacement, and nephrotic syndrome (proteinuria >3.5 g/d, hypoalbuminemia <30 g/L, and potentially accompanied by dyslipidaemia and oedema). Secondary FSGS may present similarly to primary FSGS, but lesions will be accompanied by an FSGS-causing pathophysiologic process such as a hyperfiltration injury or diabetes. Genetic FSGS occurs in patients who have podocyte or glomerular basement membrane protein mutations. There are also cases of FSGS with no identifiable cause and absence of nephrotic syndrome.1
As in IgAN, persistent proteinuria in FSGS is a risk factor for progressive kidney failure, with severity of proteinuria being associated with a faster time to kidney failure. For instance, while less than 15% of patients with non-nephrotic proteinuria progress to kidney failure in 10 years, at least 50% of patients with nephrotic proteinuria (>3 g/d) progress to end-stage renal disease in 5–10 years, with an average time to end-stage renal disease in patients with high levels of proteinuria (>10–14 g/d) of 2–3 years.40
Kidney survival is greatly improved when proteinuria is controlled. For example, in one study (n=338) where complete (≤0.3 g/d) or partial (>50% reduction to <3.5 g/d; or reduction to <2 g/d) proteinuria remission was achieved in 26% and 25% of patients, respectively. Kidney survival was significantly better at 5, 10, and 15 years follow-up compared with no remission.41
However, patients with FSGS with only PR are more likely to have poorer kidney outcomes, as evidence in a study where 52% of 117 patients with PR relapsed after a median 7 months, compared with 36% of 55 patients with complete remission who relapsed after a median time of 20 months. Relapse in the PR group was significantly associated with worsening kidney function (p=0.03) and a higher risk of kidney failure compared with patients who achieved PR with no relapse (hazard ratio: 2.90; 95% confidence interval: 1.09–7.72; p=0.03).42
Griffin observed that it is of particular interest to stratify further patients who achieve PR to understand the factors associated with better long-term survival. Data on 466 well-characterised children and adults with FSGS and proteinuria across a range of ethnic groups revealed that, while the conventional definition of PR was UP/C <3.5 g/g and a 50% reduction in UP/C, a more robust FSGS PR endpoint (FPRE) of UP/C 0.3 to <1.5 g/g and a ≥40% reduction in UP/C was associated with a better long-term outcome and reflects an earlier predictor of kidney survival in FSGS.43 Griffin noted that this is important in the context of clinical trials because “what we want when assessing new agents is good surrogate markers that will predict long-term outcomes for our patients.”
The FPRE has been further validated in a trial of 270 paediatric and adult patients with FSGS and nephrotic-range proteinuria (≥3.0 g/g), where lower proteinuria levels were associated with a 13-year extended median time to kidney failure or death. Here, reported Griffin: “In a retrospective analysis of an observational study, it was found that there were no differences in long-term outcomes between patients having a complete response and those with the robust measure of PR [FPRE], and so these were compared with patients who did not have a PR as defined by these criteria [<1.5 g/g and ≥40% reduction from baseline]. By grouping patients in this way, a significant benefit is seen for those patients who achieve either of these endpoints [complete remission and/or FPRE], compared to those patients who do not.”44 During the panel discussion, Griffin conferred how the FPRE can also be used in the clinic as a measure to inform patients as to whether they are more or less likely to progress to kidney failure.
Despite recent advances, there remains an unmet need in FSGS for more efficacious treatments with a favourable safety profile. According to KDIGO recommendations, ACEis and ARBs are standard of care along with first-line high-dose oral glucocorticoids or calcineurin inhibitors, despite the lack of evidence from randomised controlled trials.1 As long periods of immunosuppressant therapy are required,40 there is a significant risk of toxicity.1 Further, relapse is common with all current therapeutic options, and a substantial number of FSGS patients do not achieve proteinuria remission and remain at a high risk of progressive kidney disease.1,42,45,46
To advance therapeutic options in FSGS, two Phase III trials are currently investigating novel therapies. Sparsentan is being investigated in the DUPLEX study, which has a PIE of the proportion of patients achieving a UP/C ≤1.5 g/g and a >40% reduction from baseline in UP/C (FPRE) at Week 36.47 In addition, the C-C chemokine receptor type 2 inhibitor DMX-200 is being investigated in the ACTION3 study, which has a PIE of percent change in urine UP/C (based on 24-hour urine collection) from baseline to Week 35.48
Evidence for the Dual Role Endothelin 1 and Angiotensin II in Proteinuria and Chronic Kidney Disease Progression in IgA Nephropathy and Focal Segmental Glomerulosclerosis
Pierre-Louis Tharaux
The peptide ET-1 is the most biologically relevant endothelin to kidney physiology. It is a highly stable molecule and an extremely potent vasoconstrictor with long-lasting effects.49 ET-1 is produced most prominently in the kidney with G-protein coupled endothelin receptors B ubiquitously expressed therein, depending on location.50 Both receptors are expressed in the afferent and efferent arterioles, and podocytes; mesangial cells primarily express ETAR; and, the proximal tubule, cortical and inner medullary collecting ducts, thick ascending limb, and vasa recta capillary primarily express endothelin receptor B.49,50
Tharaux discussed how experimental evidence indicates that podocyte functional cytoskeletal dynamics are altered when stimulated by ET-1, promoting cell detachment and loss of podocyte foot processes (Figure 3).51-54 The podocyte can also produce heparanase, an enzyme that cleaves the endothelial glycocalyx, the first layer of the glomerular filtration barrier that ensures selectivity against proteinuria in the glomerulus. Endothelial cells also react to enhanced ET-1 signalling with resulting production of reactive oxygen species, the opening of endothelial junctions, and degradation of the endothelium.51-54 As discussed in previous sections, ET-1 can act in tandem with Ang II, leading to the amplification of the ongoing inflammatory cytokine response, direct podocyte injury, and vascular dysfunction, including excessive glomerular capillary pressure. These actions can worsen glomerular injury and proteinuria, driving tubular damage and fibrosis, culminating in progressive disease.14,52
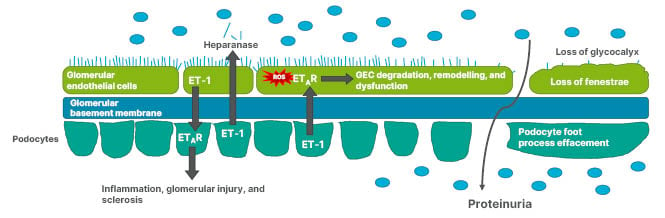
Figure 3: Podocyte and glomerular endothelial cell interaction via endothelin-1 can result in glycocalyx loss and altered podocyte cytoskeletal dynamics.
ET-1: endothelin 1; ETAR: endothelin A receptor; GEC: glomerular endothelial cells; ROS: reactive oxygen species.
ET-1 promotes chronic kidney disease via activation of ETAR (Figure 3),52-54 and several studies have suggested a role for ET-1 in FSGS and IgAN. For example, urinary ET-1 is elevated in primary FSGS,55 as is ETAR staining in kidney cells, primarily in the glomerular endothelial cells.56 In IgAN, a kidney biopsy study revealed that ET-1 expression in glomeruli and proximal tubular epithelial cells was significantly greater in patients with higher-grade (≥2 g/24 hrs) than lower‐grade (<2 g/24 hours) proteinuria or controls.57 This has been correlated with proteinuria and 1-year progression in IgAN.57,58 Studies have also shown that in patients with IgAN, neutrophils stimulate mesangial cell production of ET-159 and monocytes have increased ET-1 expression.60
Targeting of the ET-1 pathways may lead to proteinuria reduction. For example, in a murine model of IgAN, ETAR antagonism reduced proteinuria and downregulated pro-inflammatory, pro-fibrotic, and pro-sclerotic pathways.61 In FSGS and IgAN, combined RAS blockage and ETAR inhibition has been shown to achieve a substantial anti-proteinuric effect.62,63 Clinical evidence (as provided by the RADAR and SONAR studies of patients with proteinuria plus diabetic nephropathy or Type 2 diabetes, respectively) shows that selective ETAR antagonism with atrasentan, on a background of maximal RAS inhibition, can significantly decrease proteinuria64 and reduce the risk of kidney failure.65
As a DEARA, sparsentan has regions with affinity for both ETAR and AT1R, and can bind individually to either receptor to inhibit intracellular signalling.14,66 In the PROTECT study in patients with IgAN, interim results showed that sparsentan reduced proteinuria to a greater extent than the AT1R antagonism alone with irbesartan (49.8% versus 15.1% reduction from baseline in UP/C at Week 36; p<0.0001).67 In the DUPLEX study interim results, a greater proportion of patients with FSGS achieved FPRE (UP/C ≤1.5 g/g and >40% reduction in UP/C from baseline) with sparsentan than with irbesartan (42% versus 26% of patients at Week 36; p=0.0094). Preliminary safety analysis found a profile similar to irbesartan.68 In both studies, preliminary reviews of interim data showed that the safety profile of sparsentan was consistent with previous observations, that it was generally well tolerated, and that no new safety signals had emerged.67,68
Conclusion
The presentations in this symposium highlighted how proteinuria is an important measure in IgAN and FSGS, with elevated protein levels being the single strongest prognostic indicator for disease progression in both.15 Complete or partial remission of proteinuria is associated with more favourable outcomes18,41, and treatment should be aimed at achieving these goals.1 However, there are still a number of patients who remain at high risk of disease progression despite first-line treatment approaches.21,22 In FSGS, application of the novel measurement of FPRE is associated with better long-term outcomes and allows for earlier prediction of kidney survival,43,44 while in IgAN, the IgAN Prediction Tool is recommended to aid prognosis.1,16,17
Several Phase III trials are investigating a range of novel therapeutic approaches to address proteinuria in IgAN and FSGS, including those that target ET-1 and Ang II,27,28,30-32,47 which act in tandem to worsen glomerular injury and proteinuria.9,13,14,39 After a paucity of treatments for these conditions, it is hoped that new therapies will add to treatment choices.








