Abstract
Viral haemorrhagic fever (VHF) viruses cause infectious diseases that are of public health importance. Billions of people from all over the world are at risk of exposure to infection from these viruses with epidemics or outbreaks occurring in both endemic and non-endemic areas. The VHF viruses are largely neglected but are re-emerging as real threats to global health. While the more dramatic clinical presentations (such as bleeding and vascular collapse) are well known, the contribution of features that involve the central nervous system (CNS) to mortality and morbidity are not fully recognised. The diagnosis of VHF in febrile patients with CNS-related features, but who do not present with characteristic bleeding, may be missed or delayed because of a low index of suspicion. The proper evaluation of such patients with CNS disease is hampered by the dearth of neurologists and investigational tools, and weak health systems in resource-limited settings where most of the VHF viruses are endemic. Addressing these constraints would require investment in capacity building and infrastructural development in the affected countries.
INTRODUCTION
Viral haemorrhagic fever (VHF) viruses are a group of single-stranded RNA pathogens that largely produce acute febrile syndromes that may include bleeding from mucosal and other body surfaces in affected individuals. The VHF viruses of public health importance are usually associated with multi-organ dysfunction that may involve the central nervous system (CNS) in severe cases, with consequent increases in morbidity and mortality.
Outbreaks of cases may occur both in endemic1,2 and non-endemic areas.3-6 As a group, the VHF viruses contribute significantly to the overall global disease burden7-13 and have a negative socio-economic impact on the lives of the people in the affected communities. Some of the viruses may even be used for bioterrorism.14 Unfortunately, these viruses and the diseases they cause have remained largely neglected, leading to avoidable suffering among the people of the regions where they are endemic. Significant responses to VHF outbreaks get national or international attention usually only when a severe epidemic or pandemic occurs, as was the case with recent Ebola virus disease (EVD) in West Africa. Even then, the global response was slow in coming,4 which likely contributed to the severity of the outbreak and the consequent huge human and economic toll that ensued.
The many sporadic cases of VHF, with varying degrees of clinical severity, are generally under-reported. These aggregate to assume significance in terms of the annual total number of cases and the morbidity that may occur. For example, it is well known that even subclinical or mild Lassa fever infection can lead to sensorineural hearing loss of varying degrees of severity,15 leading to educational and socio-economic implications. There is little or no assessment of the true burden of hearing impairment due to Lassa fever in all the areas endemic with the disease, even though estimates put the occurrence of hearing disability attributable to Lassa fever at about one-third of infected patients.
The VHF viruses, especially Lassa fever and Ebola, are rarely mentioned as important causes of CNS disease in the literature. This may be because of a paucity of information, partly due to incomplete evaluation of affected patients and the under-reporting of cases that are not associated with bleeding. The recent outbreak of EVD in West Africa illustrates the difficulties encountered in the proper evaluation of patients with VHF.3 However, many studies,16-19 including the recent ones on EVD,20,21 indicate that VHF viruses are aetiological agents of CNS disease. This paper discusses the contribution of VHF to CNS disease, highlighting the spectrum of CNS manifestations, and the challenges encountered in the diagnosis, evaluation, and treatment of patients who present with CNS features in resource-limited settings.
BRIEF OVERVIEW OF NEUROLOGICAL FEATURES OF VIRAL HAEMORRHAGIC FEVERS OF PUBLIC HEALTH IMPORTANCE
Ebola Virus Disease
The main clinical features of this disease are vomiting and diarrhoea.3,4,22,23 CNS features include encephalopathy, acute meningitis, meningoencephalitis complicated by cranial neuropathies, and radiculopathy.20 The mechanism of neurological disease may be metabolic, immune-mediated, or neurocytopathic.20,21,24,25
Marburg Viral Haemorrhagic Fever
Neurological features may occur in the severe stage of the disease, and consist of irritability, confusion, and aggression.26 The mechanism of CNS disease may include electrolyte imbalance and metabolic encephalopathy from liver and renal failure.
Lassa Fever
A spectrum of neurological manifestations occurs in different phases of the illness.16-18,27-32 This includes aseptic meningitis, sensorineural deafness, encephalitis, encephalopathy, ataxia, and subacute or chronic neuropsychiatric syndromes. Coexistence with malaria leads to an increased frequency of CNS features.16 Metabolic,29,30 direct neurocytopathic,18,28 or immunologic15,29 are possible pathogenetic mechanisms of CNS disease.
Dengue Fever
Neurological features, the mechanisms of pathogenesis which include direct neurotropic effect, metabolic derangement, and post-infectious immune-mediated processes, may involve all parts of the nervous system, and include clinical manifestations such as headache, insomnia, irritability, encephalopathy, seizure, encephalitis with focal neurological deficit, motor deficit, myelitis, encephalomyelitis, myositis, polyradiculoneuritis, neuromyelitis optica, mononeuropathy, polyneuropathy, altered levels of consciousness, and stroke.33-35
Rift Valley Haemorrhagic Fever
CNS disease in Rift Valley Haemorrhagic Fever includes features such as retro-orbital headache, temporal visual loss without retinopathy, retinitis, visual hallucinations, nuchal rigidity, meningeal irritation, confusion, seizure, hyperreflexia, ataxic gait, blindness, quadriparesis, transient alteration in level of consciousness, stupor, coma, hypersalivation, teeth-grinding, locked-in syndrome, and choreiform movement of upper limbs.36,37 The mechanism of CNS disease is cytopathic, with the brains of patients showing areas of focal necrosis with infiltrates of mainly lymphocytes and macrophages, and perivascular cuffing on histology.36
Crimean-Congo Haemorrhagic Fever
Neurological features include nuchal rigidity, confusion, irritability, mood swings, lassitude, and depression.38 The pathogenetic mechanism of CNS disease may be metabolic as a consequence of liver and renal failure, leading to encephalopathy.38
Argentine Haemorrhagic Fever
Neurological features associated with Argentine haemorrhagic fever include tremors of the tongue and upper extremities, cerebellar signs, and confusion.10,39 However, cerebrospinal fluid (CSF) findings may be normal.39 The mechanism of the cerebellar signs occurring in the convalescent period may be immune-mediated.40
Omsk Haemorrhagic Fever
CNS manifestations occur following a relapse in 30–50% of cases with this disease. Common CNS features include permanent headache and meningism; permanent but rare CNS complications are hearing loss and behavioural, psychological, or psychiatric challenges associated with neurological dysfunction.12 The mechanism of CNS disease is considered to be neurocytopathic with associated severe breaching of the blood–brain barrier in some cases.12
Yellow Fever
CNS manifestations of yellow fever include headache, agitation, delirium, convulsions, stupor, and coma. These features are probably due to metabolic encephalopathy resulting from extensive hepatic necrosis and renal failure, even though brain oedema, increased CSF pressure, and elevated CSF protein may be seen in severe cases.41
EVALUATION OF PATIENTS WITH CENTRAL NERVOUS SYSTEM INFECTIONS DUE TO VIRAL HAEMORRHAGIC FEVER VIRUSES
The clinical evaluation of the patients with CNS infectious disease due to VHF viruses begins with an analysis of the patient’s history, followed by a detailed physical examination when practicable. Fever, headaches, and neck stiffness without a significantly altered level of conscious would suggest aseptic meningitis, while abnormal behaviour, confusion, seizures, and significantly altered mental status, in addition to fever and headache, would increase the likelihood of encephalitis. A physical examination finding of nuchal rigidity is a classic feature of meningeal irritation, and the presence of Kernig’s and Brudzinski’s signs may add support to the clinical impression of meningitis. In viral encephalitis, physical examination may reveal confusion, disorientation, irritability, ataxia, tremors, varying degrees of profound altered levels of consciousness, and focal neurological deficits.
If there are no contraindications for lumbar puncture, a CSF sample should be obtained and examined for its physical, biochemical, and microbiological characteristics. The essential CSF findings for both aseptic meningitis and encephalitis caused by viruses include pleocytosis (mainly mononuclear cells), normal or decreased glucose levels, and normal or increased protein levels; Gram’s stain is negative and culture is sterile. Further evaluation of the CSF would be determined by the prevailing epidemiological characteristics, and may include CSF polymerase chain reaction (PCR), serology, and viral culture (Table 1). Blood culture, serum serology, and blood PCR may be relevant depending on the suspected aetiological agent.
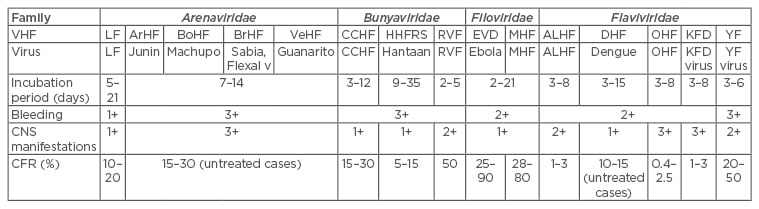
Table 1: Viral haemorrhagic fevers with relative frequencies of bleeding and central nervous system features.1
1+ = not frequent; 2+ = frequent; 3+ = more frequent.
VHF: viral haemorrhagic fever; LF: Lassa fever; ArHF: Argentine haemorrhagic fever; BrHF: Brazilian haemorrhagic fever; BoHF: Bolivian haemorrhagic fever; VeHF: Venezuelan haemorrhagic fever; CCHF: Crimean-Congo haemorrhagic fever; HHFRS: Haatan haemorrhagic fever with renal syndrome; RVF: Rift Valley haemorrhagic fever; EVD: Ebola virus disease; MHF: Marburg haemorrhagic fever; ALHF: Alkhumra haemorrhagic fever; DHF: Dengue haemorrhagic fever; OHF: Omsk haemorrhagic fever; KFD: Kyasamur forest disease; YF: yellow fever; CNS: central nervous system; CFR: case fatality rate.
In addition, a patient with suspected acute viral encephalitis should undergo neuroimaging and electroencephalogram examinations to distinguish between focal or diffuse abnormalities of brain parenchymal involvement. In some instances of viral encephalitis, neuroimaging may be normal. If CSF PCR is not diagnostic and the patient’s clinical status continues to deteriorate, with magnetic resonance imaging (MRI) scans showing focal parenchymal involvement, a brain biopsy may be justified to make a definitive diagnosis, and guide specific treatment.42
Relevant investigations to exclude metabolic disturbance, haematological abnormalities, sepsis, and fluid or electrolyte imbalance include full blood and differential counts, erythrocyte sedimentation rates, C-reactive protein, blood sugar, serum electrolytes, urea and creatinine, liver function tests, blood cultures, serum amylase, and urine analysis. It is important to remember that both encephalopathy and encephalitis can coexist.
While evaluating VHF CNS infections, especially when bleeding is not a feature, important differential diagnoses of infectious origin are the same for non-VHF causes, and include partially treated bacterial meningitis, herpes viral encephalitis, lymphocytic choriomeningitis virus infection, mycobacteria, neurosyphilis, Listeria spp., Brucella spp., Mycoplasma spp., Coxciella spp., Rickettsia spp., Leptospira spp.,42 and malaria, causing conditions that, when diagnosed, have effective treatments.
MANAGEMENT OF VIRAL HAEMORRHAGIC FEVERS
Suspected and confirmed cases should be managed in isolation facilities where care should be taken to prevent truly negative cases from eventually becoming infected by laboratory confirmed patients. For all VHF, supportive care is important and may be the only treatment option. Fluid and electrolyte replacement, including blood transfusion when indicated, are essential.2
Specific therapy is available for some VHF. The antiviral drug ribavirin shows varying degrees of effectiveness against the Lassa virus, Junin virus, Hantaan virus, Rift Valley haemorrhagic fever virus, and Crimean-Congo haemorrhagic fever virus; ribavirin is particularly effective in Lassa fever when administered within 6 days of the onset of the illness.43 The use of commercially produced human monoclonal antibodies and novel experimental drugs have shown promise in the treatment of EVD20,44 and may be the mainstay of specific treatment in the near future. The administration of convalescent serum is indicated in Argentine viral haemorrhagic fever,10 and has been found to be useful against other VHF viruses,20,44 especially in those without specific antiviral therapy. It was the only effective means of treating VHFs in the past,12,45 and still has relevance today.20
Multi-organ disease would require management in intensive care settings where appropriately trained medical staff are available. The EVD survivors infected in West Africa during the recent Ebola outbreak, and treated in the UK and USA, have shown that even in patients with very severe disease, survival is possible when the appropriate infrastructure and medical expertise are available.20,44
Prevention and Control Measures
Knowledge of the vector(s)/host(s) and the mode(s) of transmission of the specific virus provide strategies for prevention. For the VHF viruses, avoiding primary or secondary exposure is of paramount importance. Once an index case is identified, standard infection control measures must be started immediately.46
Isolation of infected humans in well-equipped and staffed designated isolation facilities is ideal. The education and re-education of healthcare personnel on measures to prevent nosocomial infections cannot be overemphasised. Post-exposure prophylaxis may be applicable, for example in Lassa fever exposure.47 Vector control measures applied appropriately may reduce the rates of transmission and disease. Unfortunately, many VHF viruses currently do not have effective vaccines against them; effective vaccines are available for yellow fever, Omsk haemorrhagic fever, and Dengue fever. The devastating consequences of the recent EVD in West Africa has made urgent development of effective vaccines against the VHF viruses of public health importance imperative.
Challenges in Diagnosis, Evaluation, and Treatment
Late presentation to hospital is a common phenomenon in resource-limited areas. The varied and non-specific nature of the early stages of these viral diseases with early symptoms and signs shared by common endemic diseases,1 the penchant for self-medication, the patronage of patent medicine sellers, the use of traditional remedies, the consultation with faith healers, the scarce number of healthcare facilities in rural communities, the difficulty in accessing the available healthcare facilities, and the myths and false perceptions surrounding these diseases, all contribute to late presentation that leads to increased mortality and morbidity. CNS diseases typically occur in the late stages of VHF illnesses.
The non-specific nature of the clinical features of most VHFs makes early clinical diagnosis difficult, thus requiring a high index of suspicion. In some instances, even in late stages of the disease, bleeding may not be a clinical feature. Many patients may be treated first for malaria, and common causes of diarrhoea, resulting in delay in early diagnosis, and the potential for nosocomial spread if preventive control measures are not observed routinely.48,49 Early case recognition, rapid laboratory diagnosis, and early commencement of supportive and specific therapies, where applicable, may improve prognosis.3
Furthermore, bleeding is not present in all cases of VHF,1 and is also not specific for VHF. Strain variation of the virus in different regions of the world and within individual countries, coupled with host factors, may influence the clinical features in different areas, and may not allow for generalisation in the application of clinical diagnostic criteria. Unfortunately, even in areas endemic for VHF, many healthcare workers may not be familiar with the clinical features of the major VHF, particularly when bleeding is absent, resulting in missed or delayed clinical diagnosis.49 This may result in devastating consequences to patients and healthcare workers.45,50 Healthcare workers must be familiar with the existing diagnostic criteria while efforts at improving them continue. VHF should be in the curriculum of medical education in Africa and other regions where outbreaks occur; practising healthcare professionals should be encouraged to have continued medical education on VHF.
Even though good progress has been made in the development of local infrastructure and manpower in West Africa, the number of available laboratories capable of effectively making a definitive diagnosis of VHF is still grossly inadequate.9,51 More laboratories, including well-equipped mobile laboratories that can be deployed to outbreak areas quickly, are needed. It is also of critical importance to urgently develop rapid diagnostic test kits effective in making field or bedside diagnosis of VHFs. Fortunately, a rapid diagnostic test has been shown to be effective in diagnosing Lassa fever in Sierra Leone.51 It is hoped that this breakthrough achievement can be replicated with other VHFs.
Of concern is the highly contagious nature and high fatality rates associated with of some of the major VHF, which may pose limitations to the evaluation of infected patients, as it might be too risky for researchers or clinicians to conduct full clinical evaluation and use modern investigational tools. Even where the opportunity is present for better evaluation, especially for viruses with relatively low secondary infectivity rates, the lack of infrastructural and manpower resources in resource-poor countries is a serious hindrance.3
The apparent global neglect of these re-emerging diseases has delayed the development of effective vaccines against most of the VHF viruses of public health importance. The recent EVD outbreak in West Africa showed clearly how unprepared the world is in responding to significant outbreaks of severe VHF epidemics. Perhaps financial considerations may partly explain why pharmaceutical companies are probably not keen on putting resources into vaccine development. The fact that most of the VHFs are in developing countries may also contribute to the palpable apathy towards intensive VHF vaccine research.
DISCUSSION
Many studies10,16-18,24,25,27-29,31,33-39,41 have attempted to elucidate the varied CNS manifestations occasioned by VHF virus infection in the acute, convalescent, and long-term phases of illness. However, the recent outbreak of EVD in West Africa, and the subsequent post-infectious CNS syndromes20,21 encountered show that the clinical spectrum of EVD as it relates to the CNS may still be dynamic. Furthermore, the experience at the Irrua Specialist Teaching Hospital, Irrua, Nigeria,16-18,27 would suggest that the clinical spectrum of CNS involvement in Lassa fever and the effect on outcome may be underestimated, possibly grossly under-reported, and also still dynamic. This might indicate that with advancement in case recognition, diagnostic, and therapeutic techniques, the spectrum of clinical features, especially neurological manifestations might still broaden.
CNS involvement does not equate to higher mortalities in all cases. There are differences in mortality between different families (e.g. Arenaviridae versus Flaviviridae) and within family members (e.g. Flaviviridae: Omsk haemorrhagic fever versus yellow fever) (Table 2). The reason is probably due partly to the types of associated organ systems involved, and how severely affected the organ is. For example, gastrointestinal symptoms, bleeding, and shock are prominent in Filovirus infection; while hepatic failure is usual in yellow fever. Furthermore, viral load, viral strain, initial dose of inoculum, route of infection, and host factors may play significant roles in determining the severity of infection.
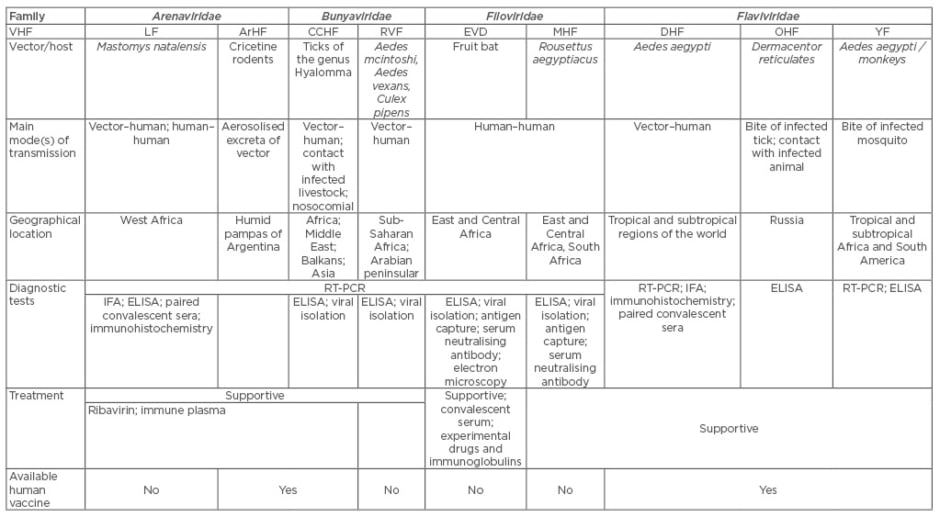
Table 2: Major viral haemorrhagic fevers: epidemiological characteristics.1
VHF: viral haemorrhagic fever; LF: Lassa fever; ArHF: Argentine haemorrhagic fever; CCHF: Crimean-Congo haemorrhagic fever; RVF: Rift Valley haemorrhagic fever; EVD: Ebola virus disease; MHF: Marburg haemorrhagic fever; DHF: Dengue haemorrhagic fever; OHF: Omsk haemorrhagic fever; YF: yellow fever; RT-PCR: reverse transcription polymerase chain reaction; IFA: immunofluorescence assay; ELISA: enzyme-linked immunosorbent assay.
In VHF that cause severe bleeding, the pathogenic mechanism involves abnormalities in haemostasis and microvascular instability leading to shock syndromes in severe cases.1 The mechanism of CNS disease in VHF is not clear-cut, as not one mechanism fits all VHFs and the spectrum of neurological features associated with them (metabolic, cytopathic, and immune-mediated mechanisms) have been variously postulated with sometimes convincing arguments. Metabolic encephalopathy from multi-organ involvement may explain the CNS disease in many instances. However, clinical and laboratory features indicative of breach of the blood–brain barrier27,31 and infection of different regions of the grey areas of the brain parenchyma20,21,25,36,52 would suggest direct effects of the virus. Experimental animal models have lent support to the possible neurotropic effect of some VHF.24,53 VHF viral particles have been recovered from the CSF20,25,31 and from brain parenchyma tissues of humans.54 Furthermore, electroencephalogram and MRI findings have shown evidence of encephalitis in some cases.20,21,52 Support for an immune-mediated mechanism comes from neurological manifestations occurring during the convalescent or post-infectious periods of infections.10,15,20,21,29,39
CNS manifestations usually indicate late stages of the VHF disease with consequent increases in mortality.17,30 Reported CNS features in VHFs include meningitis, seizures, irritability, tremors, confusion, disorientation, cranial nerve dysfunction, ataxia, neuropsychiatric syndrome, and altered levels of consciousness, including coma.10,17,20,27,36,38,39,41 These features are due to meningeal irritation from inflammation of the meninges and brain parenchymal injury, alone or in combination.
However, the degree or frequency of CNS involvement differs amongst the various VHF (Table 2). Some VHF viruses have strong predilection for the CNS in the natural course of the disease while others are not as strong.1 For example, the prevalence of CNS disease in Omsk haemorrhagic fever may be between 30% and 50%12 and the frequency ≤40.6% in hospitalised Lassa fever patients.29 Therefore, the incidences of aseptic meningitis and encephalitis among the VHF viruses would be expected to vary. Even though CNS infections are regarded as serious diseases, meningeal involvement alone causing aseptic meningitis may have a good prognosis, for example in Lassa fever.27 Reported cases of encephalitis without significant involvement of other organ systems are rare; it is therefore difficult to ascertain the impact on outcome when the VHF viruses infect the brain parenchyma alone. However, in general, a clinical diagnosis of encephalitis may be associated with poor prognosis.17,31,36,39 But the encephalopathy resulting from dehydration, metabolic dysfunction, and electrolyte perturbations due to blood loss, diarrhoea, vomiting, and hepatic and renal dysfunction, which may be present in patients with multi-organ disease, including the CNS, may influence the clinical course and outcome as well. Some clinical features of encephalopathy, such as disorientation, confusion, altered levels of consciousness, and coma, may be indistinguishable from features due to encephalitis, making separation of the contribution of brain injury to disease severity and outcomes in patients with multi-organ disease difficult. Even among the same families of VHF viruses and virus species, there is variation in frequency and severity of CNS disease (Table 2).1
Some patients may not have bleeding as a clinical feature, and CNS disease may be the only clinical manifestation of VHF,10,27,31 which may partly explain the reported low incidence of CNS manifestation in some VHF, because without bleeding patients may not seek medical help, and many clinicians may not suspect VHF.49
Effectively addressing these constraints would require regular awareness campaigns and health education targeted at healthcare workers and the communities, and emphasis placed on the fact that bleeding is not always present in VHF. In addition, assistance from, and collaboration with international institutions and agencies that have the expertise in dealing with these diseases is essential in developing the necessary manpower and infrastructure. Increased participation of governments, especiallyin providing the political will and enabling financial environment, is desirable.
CONCLUSION
VHF viruses have varying degrees and spectrums of CNS involvement. In some cases of VHF, CNS features form a great proportion of the clinical features and contribute significantly to mortality and morbidity. The exact burden of CNS disease on the population in endemic areas is not known. Proper evaluation of patients is hindered by weak health infrastructures in most of the regions where the VHFs are endemic. More resources, human and financial, are needed to aid in the diagnosis, evaluation, and management of patients with CNS disease caused by VHF.








