Abstract
Cerebrotendinous xanthomatosis is a rare autosomal recessive disorder that occurs due to defective bile acid biosynthesis, causing unusual cholesterol and cholestanol deposition in multiple soft tissues. It is manifested by various neurological and non-neurological symptoms. The characteristic imaging features and clinical symptoms can help to make an early diagnosis and induce timely treatment to prevent neurological sequelae. The authors present two adults with differing clinical symptoms, whose imaging provided pivotal cues in diagnosing cerebrotendinous xanthomatosis.
Key Points
1. There is a correlation between the imaging findings and neuropathologic changes observed in patients with cerebrotendinous xanthomatosis (CTX).
2. Despite the presence of characteristic clinical and imaging findings, it is important to differentiate CTX from similar conditions, as misdiagnosis can occur.
3. Prompt diagnosis and treatment are essential to prevent the neurological complications associated with CTX. Fortunately, CTX is a treatable condition, and with appropriate treatment, there is no progression of imaging abnormalities in affected patients.
INTRODUCTION
Cerebrotendinous xanthomatosis (CTX) is a unique disease with an autosomal recessive inheritance, occurring due to the accumulation of cholestanol and cholesterol, mainly in the brain, spinal cord, peripheral nerves, lungs, liver, kidneys, tendon xanthomas, and bile.1 It was first made known by Bogaert et al.2 in 1937. The pathogenesis includes abnormal synthesis of bile acid due to deficient action of liver mitochondrial enzyme sterol 27-hydroxylase.3 This leads to reduced production of cholic acid and negligible chenodeoxycholic acid, thereby lowering its negative effect on 7α-hydroxylase, which is the most limiting enzyme in bile acid synthesis. This induces an alternate pathway causing excessive accumulation of cholestanol in tissues. Also, bile alcohols are synthesised in these patients through 24- and 25- hydroxylase routes.4 CTX is diagnosed biochemically by showing definitively increased levels of urinary bile alcohols and serum cholestanol levels.5
This increases the deposition of cholesterol and cholestanol in various tissues and organs, especially in the brain and tendons, and unusually in the peripheral nerves, lungs, liver, and kidneys. As a consequence, juvenile cataracts, developmental disability, tendon swelling, and cerebellar ataxia constitute the distinguished clinical presentation of this entity. Other neurological symptoms include diminished intelligence, loss of memory, dystonia, seizures, and psychotic behaviour.6 Non-neurological features could be diarrhoea, early atherosclerosis, and reduced bone density.7,8 Early diagnosis and intervention are crucial as treatment with chenodeoxycholic acid (CDCA) is helpful to the patients, and further progress of the disease can be prevented. The authors present the ultrasound (US), CT, and MRI findings of two patients of Indian ethnicity. Both patients gave written informed consent. The purpose is to add to the literature and illustrate the classic radiological findings in patients with CTX, which is commonly erroneously diagnosed, leading to neurological deterioration and delay in treatment induction, and thus affecting the prognosis of these patients. A well-timed and accurate diagnosis is of utter importance as therapy is of extreme help to these patients, and further devastation can be stopped.9
CASE REPORT
Case I
A 19-year-old female presented with progressive weakness of bilateral lower limbs and multiple episodes of seizures over the last 15 years, with an inability to walk without support for the last 2 months. The patient also had complaints of swelling over the posterior aspect of bilateral ankles for the last 6 months. They suffered from difficulty in reading and writing since childhood, and were not able to continue school after third standard. The patient also gave a history of bilateral cataract surgery a few years ago.
On examination, the patient had cerebellar dysarthria with bilateral upper and lower limb incoordination. Deep tendon reflexes were brisk with decreased plantar reflex. Bilateral pseudophakia was also noted. A nerve conduction study showed axonal sensory-motor polyneuropathy. The patient presented to the department for an MRI of the brain. Brain MRI with cervical spine screening was done on a 3T MRI scanner. The sequences acquired were T1 weighted (W), T2W, fluid attenuated inversion recovery (FLAIR), diffusion-weighted (DWI), susceptibility-weighted (SWI), and post-contrast T1W for MRI brain; and sagittal T2 fast spin-echo, sagittal T1 FLAIR, and axial T2 fast recovery fast spin-echo.
Brain MRI detected diffuse cerebellar atrophy, and bilateral symmetrical T2/FLAIR hyperintensity with intervening hypo-intense areas in dentate nuclei and surrounding cerebellar white matter (Figure 1A and 1B). On T1W imaging, these areas appeared hypointense (Figure 1C). SWI revealed corresponding areas of hypo-intensity, which appeared hyperintense on filtered phase s/o soft calcifications (Figure 1D and 1E). No diffusion restriction on DWI or contrast enhancement was noted. Screening of the cervical spine revealed no evidence of any abnormal signal intensity within the spinal cord. Additionally, the authors conducted a non-contrast CT of the head and US of bilateral ankles. Head non-contrast CT revealed bilaterally symmetrical hypodense areas in deep cerebellar white matter (Figure 1F). On US of ankles, the authors observed fusiform enlargement of bilateral Achilles tendon with increased anteroposterior thickness, loss of normal fibrillary pattern, and multiple dark foci within the tendons (Figure 1G and 1H).
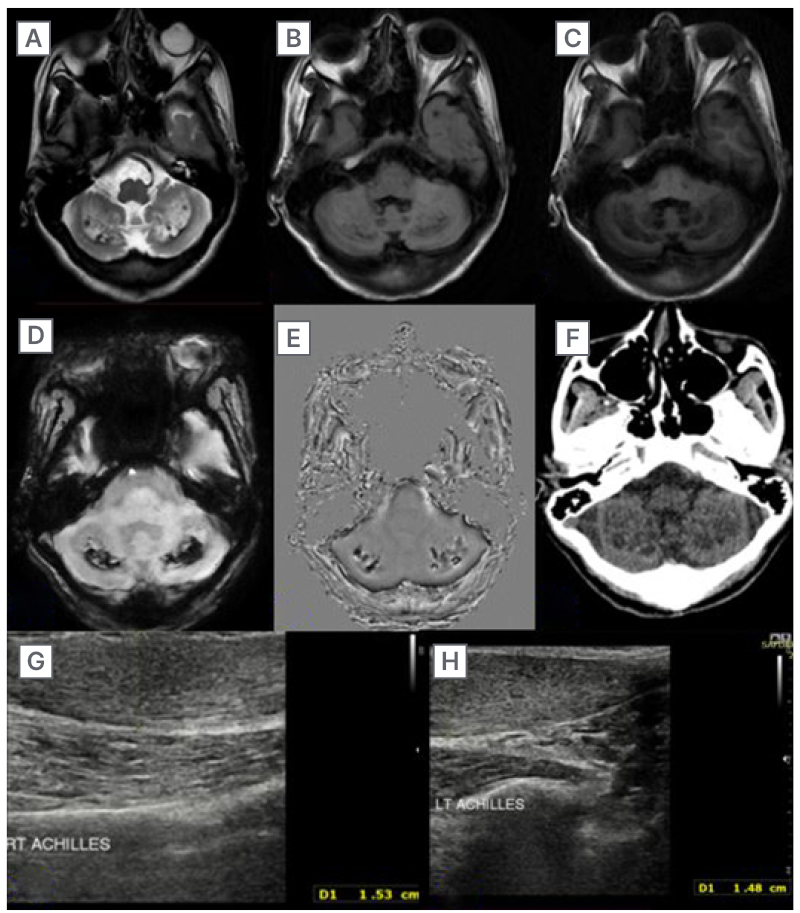
Figure 1: Imaging (MRI and ultrasound) findings of Case 1.
A–C) MRI brain T2/FLAIR images showing bilateral symmetrical hyperintensity and hypointensity on T1 weighted images. D–F) Areas of soft calcifications on susceptibility-weighted angiography and non-contrast CT head. G and H) Ultrasound sonography of the ankles revealing fusiform enlargement and multiple dark foci in the bilateral Achilles tendons.
FLAIR: fluid attenuated inversion recovery.
Case II
A 40-year-old male presented with clinical complaints of multiple soft tissue growth over bilateral ankles, elbow, knee, and dorsum of hands for many years. The patient also had cognitive and language impairment. They gave a history of unilateral cataract surgery with a diminution of vision in the contralateral eye.
On clinical examination, the patient had firm, non-tender lobulated swellings seen over the bilateral Achilles tendon, bilateral infrapatellar region, bilateral elbows, and dorsum of bilateral hands (Figure 2A–C). The biopsy of the swellings revealed diffuse sheets of foamy histiocytes in the dermis and collections of cholesterol clefts with foreign-body giant cells, suggestive of xanthoma with foreign-body giant cell reaction.
MRI brain with cervical spine screening was done on a 3T MRI scanner. The sequences performed were T1W, T2W, FLAIR, DWI, T2-weighted magnetic resonance angiography, and post-contrast T1 fast spin-echo for the brain MRI. This showed bilateral symmetrical altered signal intensity areas involving dentate nuclei and deep cerebellar white matter, which appeared heterogeneously hypointense on T1W and T2W image, with surrounding T2/FLAIR hyperintensity, which was seen extending to bilateral middle cerebellar peduncle and dorsal pons (Figure 3A–D). SWI revealed corresponding areas of hypo-intensity, which appeared hyperintense on filtered phase, suggestive of the presence of soft calcifications (Figure 3E). No diffusion restriction on DWI or contrast enhancement was noted. No abnormal signal intensity within the spinal cord was appreciated on cervical spine screening done by sagittal T2W sequence.
Additionally, the authors conducted US of bilateral ankles and bilateral ocular US. Ocular US revealed right-sided pseudophakia, and the left lens revealed an expanded lens with stippled hyperechoic contents within lens material (Figure 3F). On US of the ankles, the authors observed fusiform enlargement of bilateral Achilles tendon with increased anteroposterior thickness, and loss of normal fibrillary pattern with multiple dark foci within the tendons (Figure 3G–H).
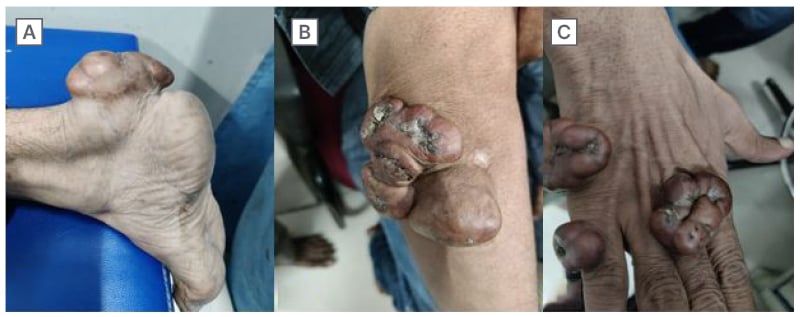
Figure 2: Clinical pictures of Case 2.
A–C) During the clinical examination, showcasing firm and non-tender lobulated swellings were observed over the Achilles tendons bilaterally, as well as in the infrapatellar regions, elbows, and dorsum of the hands bilaterally.
The diagnosis in the authors’ patients was based on clinical and radiological findings, and treatment with CDCA was started in both. After 3 months of therapeutic interval, Achilles tendon lesions decreased; however, no significant change in neurological symptoms was noted.
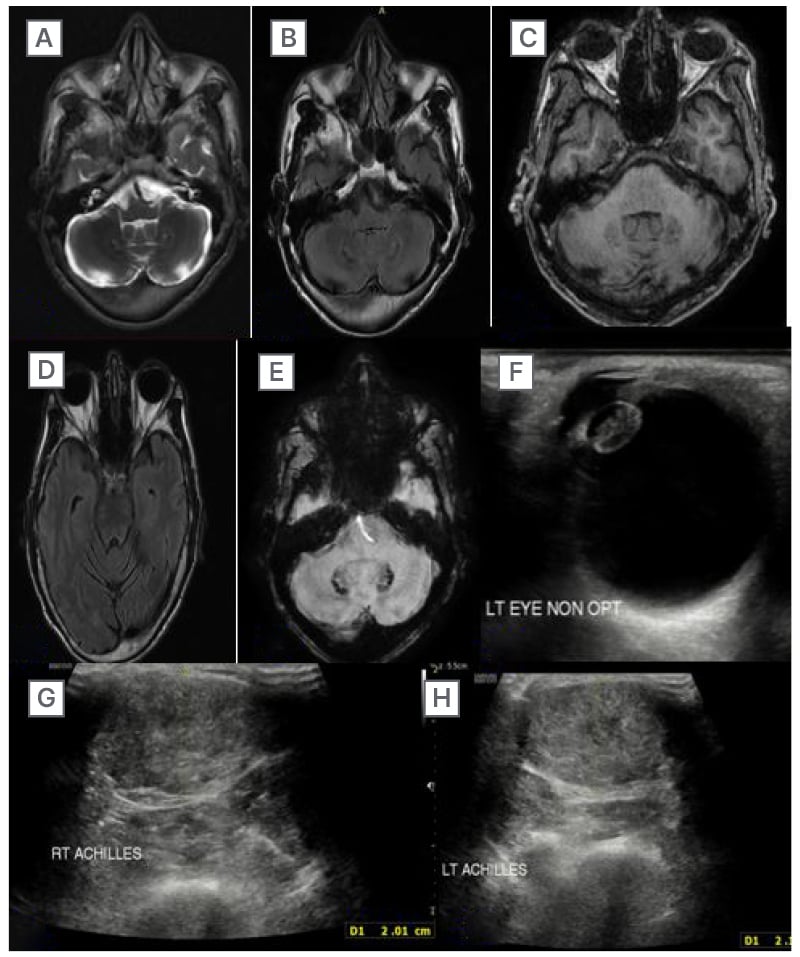
Figure 3: Imaging (MRI and ultrasound) findings of Case 2.
A–D) The MRI brain scan revealed bilateral symmetrical hyperintensity on T2/FLAIR sequences in the dentate nuclei and deep cerebellar white matter, affecting the bilateral middle cerebellar peduncle and dorsal pons. E) The susceptibility-weighted imaging scan showed the presence of soft calcifications. F) The ultrasound scan of the left eye revealed an enlarged lens with stippled hyperechoic contents. G–H) The ultrasound scan of the ankles revealed fusiform enlargement of the bilateral Achilles tendons.
FLAIR: fluid attenuated inversion recovery.
DISCUSSION
CTX is an uncommon autosomal recessive disorder due to mutations in the gene CYP27A1, on chromosome 2q33-qter, causing deficiency of an enzyme, mitochondrial sterol 27-hydroxylase, which plays a crucial role in bile acid synthesis.1 It is a widely stated mitochondrial enzyme, associated with the mitochondrial chrome P450 enzyme family, capable of catalysing multiple hydroxylation reactions in the synthesis and metabolism of cholesterol and bile acid. Its deficiency leads to the absence of negative feedback with excessive production, and build-up of cholestanol in various tissues, chiefly in the brain, lens, and tendons.10 The blood cholestanol levels are raised; however, serum cholesterol levels can stay within normal range.6
CTX shows slow progression and significant variation in age of onset and clinical presentation.11 These patients usually present at an average age of 35 years, and commonly there is a lag in the diagnosis of 16 years.12 This disease entity, however, does not interfere with the lifespan of patients. They usually present with various neurological and non-neurological symptoms. Early diagnosis and intervention are important to stop the progression of neurological deterioration.13 The usual triad of clinical features consists of over-early bilateral cataracts, tendon xanthomas, and neurological abnormalities.14 These clinical manifestations occur due to the abnormal accumulation of cholesterol and cholestanol, and include juvenile cataracts, progressive neurologic dysfunction, pyramidal and cerebellar signs, diminished intelligence, peripheral neuropathy, tendon xanthomas, chronic diarrhoea, atherosclerosis, osteoporosis, bone fractures, and respiratory symptoms.15,16
Ram et al.17 reported two cases of CTX, both showing Achilles tendon xanthomas, cataracts, diarrhoea, and mental disability. The most involved tendons are the Achilles tendon and the quadriceps tendon. The triceps, finger extensor tendons, olecranon, tibial tuberosity, and plantar surface of the foot are relatively uncommon to be involved.17 Hokezu et al.18 reported only mild cerebral atrophy on imaging, even in the cases of severe mental deterioration in eight patients diagnosed with CTX. The patients in the present study had differing clinical complaints, with one showing prominent cerebellar signs with progressive weakness, while the other patient had multiple subcutaneous swellings with cognitive impairment.
One of the authors’ patients had extensive tendon xanthomas involving multiple extensor tendons, as confirmed on histopathology and US, and another patient had bilateral Achilles involvement. Detailed high-resolution US of the Achilles tendon can be used to monitor disease progression. This technology is equally good as MRI, and still the best modality for follow-up because of its easy availability and lack of radiation. These tendon xanthomas appear hypointense on T1 and T2W image MRI, in contrast to the T1W hyperintense appearance of the normal tendon, due to the presence of internal fat. The decrease in anteroposterior diameter is indicative of a better response to the treatment. Histopathology of the tendons shows an aggregation of xanthoma cells with various, distributed lipid crystal rifts.19
Classical MRI findings are bilateral symmetrical altered signal intensity in dentate nuclei and surrounding cerebellum, which appear hyperintense on T2W image. There are widespread or localised supratentorial white matter abnormalities due to demyelination, which can also be seen in cerebral peduncles and globus pallidus. A characteristic hypointense rim along these lesions on T2W image has also been described, which is formed due to xanthomas.20,21 A few hypointense foci likely representing haemorrhage or calcification have also been noted within the hyperintense areas in the dentate nuclei and the cerebellar white matter.19 Cerebral and cerebellar atrophy is also a common finding. The authors’ patient’s non-contrast CT and MRI findings were typical and consisted of bilaterally symmetrical hypodensities on CT and hyperintense signal on T2W/FLAIR in deep cerebellar white matter.
The imaging findings, especially symmetrical cerebellar lesions, are typical for CTX; however, few of these findings, such as symmetric deep grey matter and cerebellar involvement, can be observed in other rare diseases, like Erdheim–Chester disease, Langerhans cell histiocytosis,22-25 and a few peroxisomal disorders, such as Refsum disease and adrenomyeloneuropathy.26,27 The pathogenesis responsible for central nervous system findings, as suggested by a few authors, is due to demyelination, whereas few suggest mainly a neuroaxonal process, rarefaction with resultant loss of neurons and myelin.28,29 The dentate nucleus has been reported to be most susceptible to ischaemic, metabolic, infectious, and degenerative nerve diseases.30 Other findings, like atrophy of the spinal cord, brainstem, and corpus callosum, including spinal lateral and dorsal column lesions, are quite rare.19 None of the authors’ patients revealed such a finding. The involvement of the spinal cord has also been described in a symmetrical pattern predominant involving grey matter with mild contiguous extension in the white matter. In rarer circumstances, isolated involvement of the spinal cord has been described in the form of chronic myelopathy without any cerebral or cerebellar signs.31 Common differential diagnoses of CTX are tabulated in Table 1.32-36
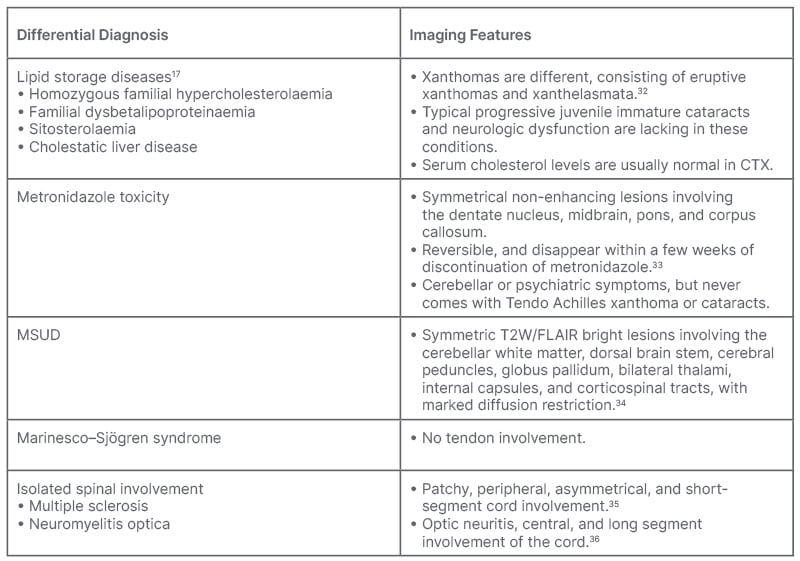
Table 1: Differential diagnosis.
CTX: cerebrotendinous xanthomatosis; FLAIR: fluid attenuated inversion recovery; MSUD: decompensated maple syrup urine disease; W: weighted.
Stelten et al.37 found that the prognosis is good if therapy is started before 25 years of age, and follow-up time should be prolonged.37 Another study found that CDCA is effective and safe, with positive results on an average of 9 months after induction of therapy.38 The mainstay for the therapy of CTX is CDCA, which holds the production of cholestanol and bile alcohols by providing negative feedback for the bile acid biosynthesis pathway.10 It is not suggested to remove the xanthomas surgically, as they might show rapid growth again.39 Therefore, it becomes essential that the disease is diagnosed at the earliest and treatment initiated to prevent neurological sequelae and complications.
Among the recent advances, magnetic resonance spectroscopy might help, as an association between N-acetyl aspartate levels and patients’ ailment has been established, and its role to evaluate the disease aftereffects needs to be further validated.20 Magnetic resonance spectroscopy has also shown lipid peaks, elevated choline, and reduced N-acetyl aspartate levels in affected areas, which suggests significant axonal and mitochondrial injury.40 CTX is a rare genetic entity, with less than 400 cases reported in the literature, and through this case report, the authors intend to emphasise the diverse imaging perspective of this entity.
CONCLUSION
CTX is an uncommon condition with typical clinical and radiological findings. Treatment is successful if initiated at an early stage; therefore, prompt identification of the disease is very important. The radiologist should be aware of the multimodality imaging approach to this entity so that prompt therapeutic intervention can be done to avoid further progression, neurological sequelae, and complications.







