Abstract
The European Committee for the Treatment and Research in Multiple Sclerosis (ECTRIMS) has been convening an annual congress for more than 30 years with the aim of facilitating communication, creating synergies, and promoting and enhancing research and learning among professionals for the ultimate benefit of people affected by multiple sclerosis (MS).1 Since ECTRIMS has been active, the landscape in the management of MS has changed beyond recognition, from the emergence of disease-modifying treatments (DMT) in 1996 to the increasing availability of new treatment options for patients with relapsing MS and primary progressive MS, and potential treatment options on the horizon for secondary progressive MS.2
Most recently, the 34th ECTRIMS conference (ECTRIMS 2018) was held in Berlin, Germany, from 10th–12th October, and welcomed >9,400 researchers, neurologists, and MS professionals from 105 countries.3,4 Common themes explored included the value of real-world data in making treatment decisions, the use of biomarkers for neurodegeneration, and the ongoing challenges of treating progressive forms of MS.4 One key highlight was a ‘Free Communication’ presentation discussing the implications of the recent 2017 McDonald diagnostic criteria for MS.5,6 The consensus was that the new guidelines provide higher sensitivity and lower specificity for the diagnosis of MS, meaning that the proportion of patients diagnosed with MS has increased by nearly 25%, at the expense of the clinically isolated syndrome (CIS) diagnosis that would have been previously made. This has ethical, legal, and potential socio-economic implications for people previously diagnosed with CIS who may now fit the criteria for MS. It should also be noted that some experts believe that the revisions to the new guidelines may force treatment to be given too early, discounting the impact of neurodegeneration and adverse events associated with exposure to DMT, which must be considered.
As it is not possible to review all areas covered during ECTRIMS 2018, three topics of particular interest have been selected for discussion in this article: 1) the management of MS in women of child-bearing age; 2) the measurement and management of disability progression; and 3) the management of long-term disease.
MANAGEMENT OF MULTIPLE SCLEROSIS IN WOMEN OF CHILD-BEARING AGE
Pregnancy in Patients with Multiple Sclerosis
It is well established that pregnancy has a clear impact on disease activity in multiple sclerosis (MS); the relapse rate decreases during the latter half of pregnancy, while MS disease activity often worsens in the post-partum period.7,8 It has also been reported that breastfeeding is associated with a 50% reduction in the likelihood of developing post-partum relapses, although some studies suggest a more modest effect.9 Importantly, bearing children does not appear to negatively impact disease course in the long term and may be associated with a delay in progression in women with relapsing–remitting MS (RRMS).9
Proactive management of MS during pregnancy can play a major part in reducing morbidity and enhancing quality of life (QoL), with neurologists having a key role in facilitating the decision-making process and alleviating any anxieties women may experience related to their disease. During a ‘Meet the Expert’ session on counselling women of child-bearing age held at ECTRIMS 2018 in association with Teva Pharmaceuticals Europe, Prof Diego Centonze (Rome, Italy) emphasised the importance of supporting women through their entire pregnancy journey. He noted that support should begin prior to conception to alleviate any fertility concerns and continue during pregnancy. Providing ongoing support for both mother and baby following delivery and/or follow-up after an unsuccessful pregnancy outcome is also a key role for the neurologist when managing women with MS.
During an educational session, Dr Melinda Magyari (Copenhagen, Denmark) reviewed the implications for cessation of symptomatic treatment during pregnancy, as well as the management of lactation post-partum, and advised that the risks of pharmacotherapeutic choices should be discussed before pregnancy.10 She also noted that new drugs and combination therapies should be avoided if possible during pregnancy and that all treatments should be withheld in the first trimester. Dr Magyari also stated that although breastfeeding does not increase the risk of post-partum relapses (and may actually be beneficial in milder disease), most women with MS will not experience an increase in disability from a post-partum relapse.10
Treatment Strategies During Pregnancy
Of major concern for women with MS considering pregnancy is the effect of their MS medication on their own health and that of their unborn child. Published in 2018, the ECTRIMS/European Academy of Neurology (EAN) guidelines provide specific recommendations for the use of DMT in pregnancy based on results from a total of 19 studies conducted until December 2016.11 During a scientific session at ECTRIMS 2018 exploring the timing of stopping and starting immunomodulatory treatment in pregnancy in MS,12 Dr Spalmai Hemat (Bochum, Germany) presented prospective registry data from 129 pregnant women showing that despite the natural protection of pregnancy, up to 50% of women treated with fingolimod (before or up to pregnancy) will experience a relapse during pregnancy. However, women who stopped fingolimod >2 months prior to the last menstrual period experienced more relapses before pregnancy and in the first trimester of pregnancy. Finally, Dr Hemat noted that although the large majority of women will not experience permanent disability, 10–20% showed a significant Expanded Disability Status Scale (EDSS) worsening (≥2) 6 months post-partum.12
Safety and Treatment Choice During Pregnancy
A number of posters at ECTRIMS 2018 reported that exposure to treatments during or immediately prior to pregnancy does not negatively impact pregnancy outcomes. In one example, Dr Kerstin Hellwig (Bochum, Germany) used data from a global pharmacovigilance database of >1,300 pregnancies to conclude that IFN β-1b exposure during pregnancy was not associated with an increased risk of spontaneous abortion or congenital anomalies when compared to the general population.13 The same author also presented a poster based on data from 76 pregnancies recorded in the German MS and Pregnancy Registry between 2011 and 2017, which demonstrated that IFN β-1b and glatiramer acetate (GA) treatment during lactation appears to have no negative influence on the important characteristics of child development, such as body length, weight, or reaching of developmental milestones. However, Dr Hellwig noted that the effect on post-partum disease activity has yet to be determined.14 A recent study describing outcomes from 5,042 pregnancies of patients treated with GA (20 mg/mL) was also highlighted at Teva Pharmaceuticals’ exhibition booth during ECTRIMS 2018.15 The rate of congenital abnormalities among women exposed to GA (20 mg/mL) during all three trimesters was similar to rates reported in the EUROCAT and MACDP databases that represent the general population.
Drs Johanna Balslev Andersen (Copenhagen, Denmark) and Yvonne Geissbühler (Basel, Switzerland) presented posters demonstrating no detrimental effect of teriflunomide or fingolimod exposure, respectively, on pregnancy outcomes. Dr Andersen reported that pregnancy outcomes from 31 women and men exposed to teriflunomide for at least 30 consecutive days prior to conception and with conception occurring up to 2 years after treatment discontinuation, or female partners of similarly teriflunomide-exposed men, were consistent with those of the general population.16 Similarly, Dr Geissbühler presented cumulative pregnancy outcome data from three registries (1,397 patients in total) to demonstrate that the prevalence of major congenital malformations in live births following fingolimod exposure during pregnancy varies depending on the data source. Gilenya® (fingolimod) Pregnancy Exposure Registry data suggest a slightly higher proportion than in the general population (which is in line with untreated MS patients), while PRIM and NSDB data suggest a rate similar to that observed in the general population.17
MEASUREMENT AND MANAGEMENT OF DISABILITY PROGRESSION
Limitations of Expanded Disability Status Scale in Monitoring Disability in Multiple Sclerosis
The EDSS has traditionally been the standard measure of disability in MS used in clinical trials, but it is used less frequently in the real-world clinical setting.18 However, because the EDSS focusses on walking and visible physical disability, it is less than satisfactory for assessing overall disease progression. In addition, clinical trial data based on the EDSS and other measures may not necessarily translate into real-world practice for a variety of reasons, including the somewhat artificial conditions of a clinical trial.
During the ‘Meet the Expert’ session at ECTRIMS 2018 in association with Teva Pharmaceuticals Europe, Prof Sven Schippling (Zürich, Switzerland) discussed the challenges of long-term disease management and the role of individualised therapy in managing MS. He noted that because of the inadequacies of the EDSS, certain important symptoms of MS, such as visual functioning, cognition, and fatigue, are being overlooked because they are not specifically sought out or graded. Prof Schippling emphasised that in the future, the stage needs to be set for long-term treatment success through a patient-centric, systematic trial-and-error approach to treatment.
Monitoring and Measuring Disability Progression
The acknowledged deficiencies of the EDSS in monitoring disability in MS mean that considerable effort is currently invested in identifying alternative methods for measuring disability progression, including changes in visual disturbance, grey matter pathology, brain volume changes, and cognitive decline. These three approaches were addressed in posters at ECTRIMS 2018.
Prof Carla Santiago-Martinez (Detroit, Michigan, USA) presented the results of a study comparing the effect of GA 20 mg daily injection (GA 20 mg QD) versus GA 40 mg three times weekly (GA 40 mg TIW) on retinal structures in 47 patients with RRMS.19 This study showed no significant differences in the integrity of the retinal structures following either regimen, but Prof Santiago-Martinez suggested that GA 40 mg TIW is the more favourable treatment for people with RRMS, because of the more convenient dosing schedule. A poster presented by Dr Francesco Crescenzo (Verona, Italy) described a study in which the progression of cerebral grey matter pathology (i.e., focal [cortical lesions] and diffuse [atrophy] damage) in patients initiating GA for RRMS was compared with that of untreated patients over 2 years.20 GA was found to reduce the development of cortical lesions and grey matter atrophy.
The third poster, presented by Prof Tjalf Ziemssen (Dresden, Germany), reported the interim results of a 2-year non-interventional study which showed that naïve patients treated with GA 40 mg TIW achieved a significant improvement of cognitive performance and patient-reported disability, as well as a significant reduction in annualised relapse rate (ARR).21 EDSS progression was also stabilised with GA 40 mg TIW (Figure 1).
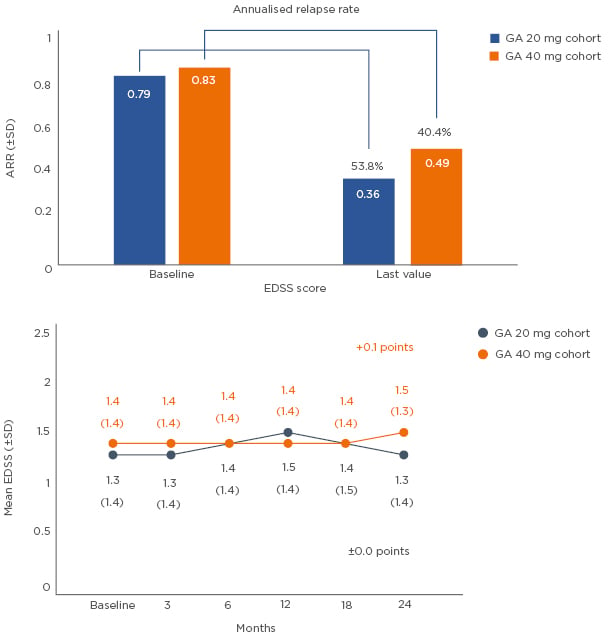
Figure 1: Annualised relapse rate* and disease progression:† GA 20 mg versus GA 40 mg cohort (interim analysis of the non-interventional study, COPTIVITY).21
*ARR was defined as the total number of relapses divided by the total number of patient-years in the study. ARR applies for 97 patients in the GA 20 mg cohort and 618 patients in the 40 mg cohort with specified number of relapses at baseline and time of last visit.
†Disease progression was defined as an increase in EDSS score ≥1 for patients with a baseline score of 0–5 and a ≥0.5-point increase for patients with a baseline EDSS score >5.
ARR: annualised relapse rate; EDSS: Expanded Disability Status Scale; GA: glatiramer acetate; SD: standard deviation.
Impact of Comorbidities on Disability
Comorbidities have a negative impact on disability in patients with MS. The relationship between DMT use and comorbidities with regard to disability in MS was explored in a poster presented by Dr Elizabeth Baraban (Portland, Oregon, USA) based on self-reported registry data collected between 2011 and 2016 from >900 patients.22 It showed patients with diabetes, depression, or respiratory disease were most likely to experience moderate or severe disability. Interestingly, disability was worst in diabetic patients, suggesting clinicians may have to factor in the patient’s comorbidities when choosing a DMT in the future.
MANAGEMENT OF LONG-TERM DISEASE
Issues of Long-Term Multiple Sclerosis Strategies
The development of optimal long-term strategies for the management of MS is of pressing concern as more treatments become available and patients with MS live longer and more active lives.23 Extensive evidence is available to support the benefits of early treatment of MS with DMT, in terms of significantly improved physical, mental, and socio-economic outcomes.24 Indeed, the current clinical consensus is that DMT should be offered as early as possible in the medical management of MS;24 however, with increasing numbers of approved treatment options for RRMS, treatment decision-making is increasingly complex for neurologists. Furthermore, controversy remains over the goals of long-term treatment and the order of priority among issues such as patient treatment goals, ongoing disability assessment, maintenance of QoL, and use of non-drug therapies.
Prof Francesco Patti (Catania, Italy) addressed these concerns during the Meet the Expert session, noting the importance of all different aspects of the disease in the long-term management of MS and observing that most patients want to participate in the decision-making process. He also commented that patients react in different ways to disease progression, with QoL not necessarily being linked to functional ability.
Treatment Strategies During Long-Term Management of Multiple Sclerosis
During a ‘Hot Topic’ session, Prof Gavin Giovannoni (London, UK) and Dr Daniel Ontaneda (Cleveland, Ohio, USA) debated whether escalation or immune reconstitution therapy is the best strategy for long-term management of MS.25 The escalation approach involves using moderately effective medications early, exposing patients to greater risk only when disease activity warrants high-efficacy treatment. In contrast, immune reconstitution therapy can carry a higher risk of infection, higher patient burden, and greater financial cost, although it can be de-risked through prophylaxis, regular surveillance for infection, and treatment switching according to risk of infection. It was agreed that clinical trial data are currently insufficient to guide decisions, although several trials (notably, HALT-MS, TREAT-MS, and DELIVER-MS) aim to answer these questions in the near future. In the ideal situation, physicians would adopt a personalised approach, guided by scores such as T2 lesion count, brain atrophy, and modified Rio score.
Long-term Treatment Outcomes
A number of posters presented at ECTRIMS 2018 provided data on the long-term outcomes of different MS treatments, including GA and ocrelizumab. For example, Prof Giovannoni presented the final 10-year results from the UK Department of Health Multiple Sclerosis Risk Sharing Scheme, established in 2002 following a National Institute for Health and Care Excellence appraisal of GA and IFN-β.26 The Risk Sharing Scheme provided eligible patients with these DMT and monitored a cohort for long-term clinical and cost-effectiveness evaluation. After a mean follow-up of 7.12 years, GA led to a 23.4% reduction in EDSS progression and 39.1% curtailment in QoL decline. Comparison of the 6-year and 10-year data showed no evidence of waning in the efficacy of GA and less than 1% change in implied hazard ratio for EDSS.
Longer-term GA data were presented by Dr Corey Ford (Albuquerque, New Mexico, USA) describing 25 years of continuous treatment from the longest prospective study of continuous DMT use in patients with RRMS.27 Mean treatment exposure was 24.6 (standard deviation: 1.3) years in the 52 patients who completed the study. Most early discontinuations due to adverse events occurred by 5 years, with 50% of patients remaining on the study at 10 years. The safety profile of GA 20 mg QD or 40 mg TIW was maintained over the 25-year study duration, with no new safety concerns arising.
Finally, Dr Thomas Leist (Philadelphia, Pennsylvania, USA) presented a 1-year interim analysis of the Phase IIIb CHORDS study, evaluating the effectiveness and safety of ocrelizumab in patients with RRMS with suboptimal response to prior DMT.28 The majority of patients showed no protocol-defined relapse (92.7%), no T1 gadolinium-enhancing (Gd+) lesions (96.4%), no new or enlarging T2 lesions (66.9%), and no 24-week confirmed disability progression (96.4%). The adjusted ARR was 0.047 and new magnetic resonance imaging activity included 5 new T1 Gd+ lesions and 177 new or enlarging T2 lesions over the 48-week trial.
OTHER HIGHLIGHTS
From a programme containing many stimulating and thought-provoking sessions related to MS, a few additional highlights deserve special mention.
Dr Klemens Ruprecht (Berlin, Germany) presented a Scientific Session on the absence of Epstein–Barr virus (EBV) seronegativity in a large cohort of patients with early MS.29 Antibodies to Epstein–Barr nuclear antigen-1 were detected in 839 of 901 patients with CIS or RRMS, while all 901 patients with CIS or RRMS in this study were EBV seropositive. The absence of EBV seronegativity in this large cohort of patients with early MS suggests EBV is a risk factor for MS and implies that negative EBV serology in a patient with suspected inflammatory central nervous system disease should alert clinicians to a possible non-MS diagnosis.
In a separate scientific session, Dr Pietro Iaffaldano (Bari, Italy) used results from the Big Multiple Sclerosis Data Network to discuss the optimal time to start treatment in patients with RRMS.30 He reported that early treatment (within 6 months of disease onset) significantly reduces the risk of reaching confirmed EDSS progression (p=0.003), with no subsequent significant comparisons between early and delayed treatment (Figure 2).
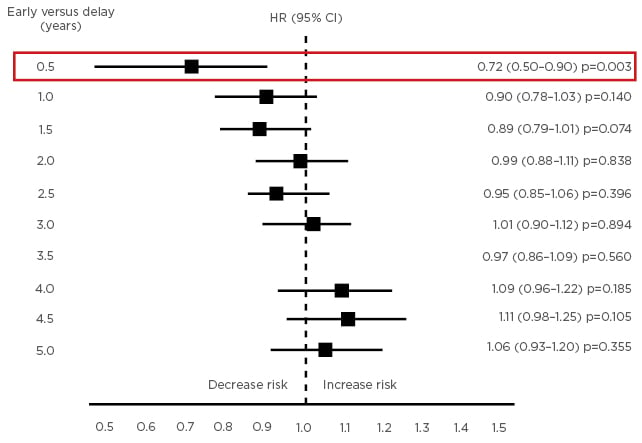
Figure 2: Risk of reaching 12 months’ confirmed EDSS progression by early versus delayed treatment start.30
CI: confidence interval; EDSS: Expanded Disability Status Scale; HR: hazard ratio.
Dr Iaffaldano concluded that the optimal time to start DMT in MS to prevent long-term disability accumulation is within 6 months of disease onset.
In regards to future MS therapies, Dr Edward Fox (Round Rock, Texas, USA) presented the final results of a placebo-controlled, Phase II multicentre study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of MS.31 All patients met the primary endpoint of >95% B-cell depletion at Week 4 (p<0.001), while at Week 48, ARR was 0.07 and 93% of patients were relapse free. Importantly, 74% of patients met the criteria for no evidence of disease activity. Following promising results from early trials, ublituximab is now in Phase III research.
Finally, Prof Xavier Montalban (Barcelona, Spain) presented the primary analysis of a randomised, placebo-controlled, Phase II study of the Bruton’s tyrosine kinase inhibitor, evobrutinib, in patients with relapsing MS.32 Evobrutinib significantly reduced T1 Gd+ lesions versus placebo in patients with active, relapsing MS during 24 weeks of treatment, while evobrutinib 75 mg once daily and twice daily significantly reduced T1 Gd+ lesions per scan versus placebo. Overall, evobrutinib was well tolerated and was not associated with serious infections, infestations, or lymphopenia.
CONCLUSION
The changing treatment landscape in MS over recent years highlights the importance of the annual ECTRIMS conference in revealing the latest updates on long-term safety and efficacy of existing therapies and new disease and treatment discoveries. With the curtains closing on ECTRIMS 2018, we look forward to ECTRIMS 2019 in Stockholm, Sweden. This year, the congress will address one of the major remaining unmet needs in MS: filling the void of effective treatment options in progressive MS.








