Abstract
Background: Lymphoma of the central nervous system (CNS), both primary and secondary, represents a very rare part of patients with non-Hodgkin lymphoma.
Methods: Exams included a neurological exam, laboratory blood tests, МRI, biopsy, and electromyography.
Results: Three different clinical cases of patients with lymphoma of the nervous system are presented. The first patient is a 44-year-old male admitted to the emergency room because of neck stiffness, with MRI data for a tumour in the left cavernous sinus area. Biopsy was performed 3 months prior to hospitalisation, showing connective tissue, partially hyalinised. Lumbar tap was performed to exclude CNS infection. Cerebrospinal fluid (CSF) examination showed lymphocytic pleocytosis, atypical cells in different phases of mitosis, and the result did not confirm lymphocytic choriomeningitis. Flow cytometric measurement (FCM) of CSF led to the diagnosis of T-lymphoblastic lymphoma of CNS.
The second patient is a 50-year-old female hospitalised in the authors’ neurological department because of lower limb weakness and decreased sensation, dysphagia, and facial nerve palsy. Brain MRI showed no abnormal lesions. Guillain–Barré syndrome was considered after performing electromyography and electroneurography. CSF showed lymphocytic pleocytosis and 47% of them were atypical. FCM of CSF helped the authors diagnose the patient with B-lymphoblastic lymphoma.
The third case presents a 63-year-old male with right sided hemiparesis and progressive cognitive impairment. Previously performed CTs and MRIs of the brain showed both hemispheres and left cerebellar peduncle diffuse lesions. Ischaemic stroke, tumour, and CNS infectious disease were considered. Most of these were excluded because CSF showed no pathological findings. Brain tissue biopsy of one of the lesions was performed, and the patient was diagnosed with diffuse large B cell lymphoma.
Conclusion: Lymphoma of the CNS is rare disease. Differential diagnoses include different conditions. FCM of CSF and biopsy could be useful in complicated patients and unknown diagnosis affecting the CNS.
Key Points
1. Lymphoma of the central nervous system (CNS), both primary and secondary, represents a very rare part of the patients with non-Hodgkin lymphoma. CNS lymphoma is associated with poor outcome, with an overall survival of 1.5 months when untreated, and a 5-year survival rate of 30% when treated.
2. These cases show the significance of varied examinations in diagnostic process.
3. Flow cytometric measurement of cerebrospinal fluid and biopsy could be useful in complicated patients with unknown diagnosis affecting the CNS.
INTRODUCTION
Primary and secondary lymphoma of the central nervous system (CNS) is a rare case of non-Hodgkin lymphoma. The World Health Organization (WHO) classifies primary CNS lymphoma as extranodal non-Hodgkin lymphoma typically confined to the brain, eyes, and cerebrospinal fluid (CSF), without evidence of systemic spread.1 Affected CNS areas could be different in each patient, as well as clinical signs and symptoms, requiring consideration of a lot of differential diagnoses. CNS lymphoma is associated with poor outcome, with an overall survival of 1.5 months when untreated, and a 5-year survival rate of 30% when treated.2
Primary CNS lymphoma (PCNSL) occurs at an incidence of 0.47 per 100,000 person-years, for 4–6% of extranodal lymphomas and 4% of newly diagnosed CNS tumours. PCNSL is more typical in males.3 The clinical and neuroimaging presentation of CNS lymphoma can be varied. The patients should not be treated for PCNSL without cytologic confirmation of diagnosis with CSF examination or biopsy of the brain. Differential diagnoses includes brain tumour, neurological infections, peripheral nerve disease, neurosarcoidosis, and vascular disease.
Most cases with PCNSL (approximately 90%) are associated with diffuse large B cell lymphomas (DLBCL), while the other 10% are T cell, mantle cell, Burkitt, or indolent B cell lymphomas.4 A 2–27% risk of developing secondary CNS disease is observed in patients with aggressive systemic non-Hodgkin’s lymphoma.5 In patients who are immunocompetent, incidence of PCNSL is approximately 51 per 10,000,000 cases per year.6
Definitive diagnosis of CNS lymphoma could depend on a positive CSF.7 New studies have showed the usefulness of flow cytometric measurement (FCM) for detecting CNS disease in B cell lymphoma.8,9 The greater sensitivity of FCM was presented in other analyses when series of CSF-stabilised samples used 4–8 colour FCM.10
CNS T-lymphoblastic lymphoma is a rare disease. Differential diagnosis is difficult, and includes varied conditions like aseptic meningitis, brainstem glioma, granulomatous angiitis, neurological infections, neurosarcoidosis, and neurosyphilis.11
The current preferable standard method for determining the tissue diagnosis of CNS lymphoma is stereotactic biopsy, since these lesions are usually deeply positioned and their resections were shown to be associated with poorer prognosis.12,13 However, histopathological evaluation of stereotactic biopsies also has some restrictions, not only because of slight sample sizes, but also due to comprehensive spectrum of differential diagnoses, including inflammatory conditions such as vasculitis, multiple sclerosis, and infection. The dense cellularity of the tumour causes isodense or hyperdense occurrence on non-enhanced CT scan and hypointense occurrence on long repetition time-weighted MRI imaging. Primary lymphoma of the CNS is supposed to be diffusely infiltrative at the time of showing, and is reckoned a ‘whole brain’ disease. The regions of disease are not visible on neuroimaging studies because they are behind a comparatively intact blood–brain barrier.14,15 For this reason, prior administration of corticosteroids (CS) represents the major diagnostic provocation. Because of the high susceptibility of lymphoma cells to CS-induced apoptosis, administration of CS can camouflage the morphology and it was even reported to cause tumours to disappear.16
Three different cases of CNS lymphoma are presented.
METHODS
The patients described were hospitalised in the authors’ clinic of neurology with different clinical presentations. Somatic and neurological exams were performed, as well as laboratory tests, including flow cytometric immunophenotyping of cerebrospinal fluid, MRI, CT scan, trepan biopsy of bone marrow and brain tissue, electromyography, chest X-ray, and coronary angiogram.
RESULTS
The described clinical cases are of patients with different neurological symptoms as an effect of CNS lymphoma.
The first case is of a 44-year-old patient admitted to the emergency department with complaints of progressively increased headache, stiffness of the neck, impaired co-ordination, nausea, sickness, and extreme sensitivity to light. Neurological examination revealed neck stiffness, double vision on the left, and downward and right limb hypoesthesia. The patient had no change in consciousness. MRI of the head was performed 4 months before admission in the neurological clinic with data showing a tumour in the area of left cavernous sinus (Figure 1A). Biopsy was performed 3 months earlier and the histology examination showed in part hyalinised connective tissue. Performed laboratory tests showed no significant abnormalities. Systemic infection and autoimmune disease were excluded by performing additional laboratory and imaging tests.
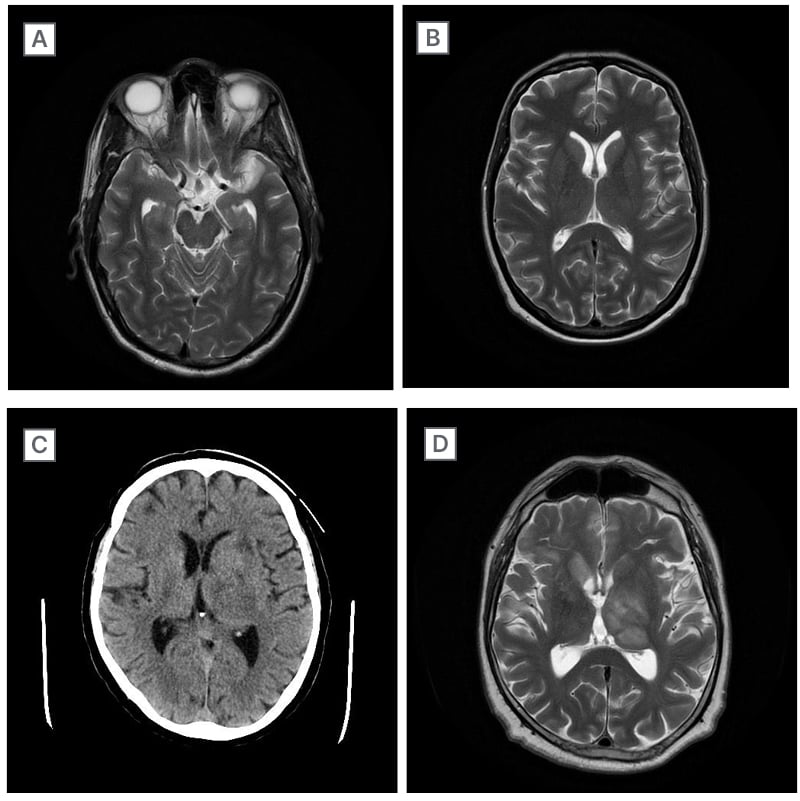
Figure 1: MRI and CT scans.
A) MRI of the brain of the first presented patient. B) MRI of the brain of the second presented patient. C) CT of the brain of the third presented patient. D) MRI of the brain of the third presented patient.
No stenosis or thrombosis of coronary blood vessels were found.
Examination of CSF showed increase in the amounts of lymphocytes. Atypical ones were observed in different phases of mitosis. CSF was tested for lymphocytic choriomeningitis, herpes simplex, varicella zoster virus (VZV), and Epstein–Barr virus (EBV). No infection agent was identified. Trepan biopsy of bone marrow was without pathological cells. FCM of CSF gave the diagnosis of T-lymphoblastic leukaemia/lymphoma. The studied materials identified: 1.5% of the CD45-positive cells were mature lymphocytes and 93% of the CD45-positive cells were T-lymphoblasts which have the following expression patterns: CD45 (+), low sCD3 (J+); partial CD2 (+); CD5 (+); CD7 (+); CD4 (+); CD8 (+); CD34 (-); CD56 (-); CD10 (-); CD19 (-); and CD20 (-). Intrathecal chemotherapy was started with high-dose methylprednisolone, vincristine, methotrexate, and cytarabine. It was combined with hyper-cyclophosphamide, vincristine sulfate, doxorubicin, plust dexamethasone.
The second case is of a 50-year-old female with complaints starting 3 days prior, including weakness in the legs, inability to walk alone, and tingling of the lower limbs from the knees down, as well as in the palms and fingers of the hands. They reported worsening of the condition this morning when they had difficulty swallowing, deteriorated speech, no taste, difficulty breathing, fatigue, and chills.
Neurological examination showed positive Kernig sign, bilateral ageusia, peripheral lesion of facial nerve on the right, and weakened muscle strength for both legs with manual muscle testing 4/5. Biceps, radial, knee, and Achilles reflexes were missing bilaterally. The patient performed dysmetria finger to nose probe with both hands and Romberg test was positive. They had decreased sensation for touch and pain in the lower extremities, and Lasègue test was positive bilaterally. Joint-muscle sensation was preserved and the patient scored 15 points on the Glasgow Coma Scale (GCS). Brain tumour was excluded by performing MRI of the brain (Figure 1B). The patient was suspected of Guillain–Barré syndrome because of the clinical presentation. EMG data showed sensory-motor axonal polyneuropathy.
CSF examination showed lymphocytic pleocytosis (403 x106/L), 47% atypical ones, and high protein level (1.98 g/L [0.00/0.45]) and lactate level (3.7 mmol/L [1.10/ 2.20]). Laboratory tests did not reveal an infectious agent (EBV, cytomegalovirus, VZV, HIV, or COVID-19). FCM of CSF was performed. In total, 122 546 cells were identified in CSF: 95.9% of all cells were CD45-positive, CD19-positive, CD10-positive, CD20-positive, CD3-negative, and CD34-megative cells with B-lymphocyte phenotype, corresponding to B-lymphoblastic lymphoma. The authors decided that lower motor neuron symptoms (missing reflexes and weakness in four limbs) could be associated with a peripheral neuropathy, mainly as a result of direct infiltration. Treatment was continued with intrathecal chemotherapy (cytarabine and methotrexate).
The third case is of a 63-year-old male with weakness in the right limbs and progressive cognitive impairment. Tremor occurred initially in their left arm, after which right arm was affected and the gait changed. Neurological examination revealed dysarthria, mild right central hemiparesis, spasticity for the left limbs, exaggerated reflexes in four limbs without pathological reflexes, right-sided hypesthesia for touch and pain, and confusion and disorientation for time and place. Several CTs and MRIs of the brain were performed with disseminated lesions in both hemispheres and left cerebellar peduncle (Figure 1C and 1D). Ischaemic stroke, tumour, and CNS infectious disease were discussed as possibilities. Laboratory tests did not reveal an infectious disease, including EBV, cytomegalovirus, VZV, HIV, or COVID-19. Examination of CSF showed no pathological changes. Treatment with steroids was started and the condition of the patient improved. Due to suspicion for lymphoma, trepan biopsy of bone marrow was performed. Fragment of a spongy bone was with hypocellular age-related bone marrow. The three haematopoietic lines were represented by maturation.
Normocellular granulocyte cells, including all maturation forms, are observed, along with normocellular erythrocyte cells. Megakaryocytes had an unincreased number and normal morphology. The reticulin network was unincreased. Bone marrow was without diagnostically significant histological changes. Patient was diagnosed after brain tissue biopsy of one of the lesions. In order to determine the histogenesis and possible primary focus, an immunohistochemical study was performed.
The result showed a CD45 and CD20-positive reaction in tumour cells; CD10-negative reaction in tumour cells; Ki67-positive reaction in approximately 80% of tumour cells; an adverse reaction to cytokerarin AE1/AE3; glial fibrillary acidic protein; S100 and synaptophysin-negative reaction in tumour cells with positive internal control in astrocytes; and CD3 and CD5-positive reaction in a small number of mature T lymphocytes.
The constellation was for DLBCL (Table 1).
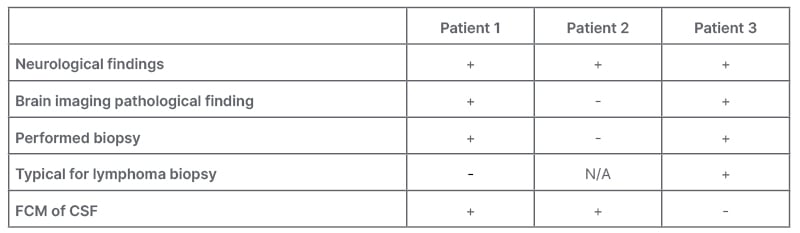
Table 1: Clinical cases comparison.
CSF: cerebrospinal fluid; FCM: flow cytometric measurement; N/A: not applicable.
CONCLUSION
CNS lymphoma is a rare but socially significant disease. Early diagnosis improves the survival rate of the patients. FCM of CSF could be beneficial to make the right diagnosis. FCM is a highly sensitive technique, efficient for finding malignant cells.17-19 It can find CNS disease before the presentation of clinical symptoms, and the routine use of FCM and cytology may enable the earlier detection of neoplasm. Many studies have proven the value of FCM for detecting CNS disease in DLBCL.20-23 More recent results of larger series of CSF-stabilised samples using 4–8 colour FCM22-24 demonstrated very little false-negative FCM results (range: 0–<1 %), further fortifying its greater diagnostic productivity with regard to conventional cytology. CSF examination should also be done in existence of neurological symptoms, in suppletion to imaging methods (MRI and CT).25 The differential diagnosis of CNS lymphoma is vast. High-grade gliomas, meningioma, granulomatous and demyelinating diseases, and vasculitis should all deserve attention.
Metastatic lesions from systemic malignancies, as well as infections within the nervous system, can also present alike, and must be excluded with a careful medical history and appropriate diagnostic tests.
CSF cytology can ensure certain diagnostic information in CNS lymphoma, and with the aid of immunohistochemical studies, it has been likely to identify atypical or neoplastic lymphocytes. Sensitivity increases when a larger volume (≥10.5 mL) is analysed and when serial CSF probes are examined.26 Sensitivity is decreased when there are delays in working, or after exposure to CS, causing cytolysis.27
CNS lymphoma often presents with atypical symptoms, which are either unrecognised or difficult to differentiate from other diseases. So, whenever manifest, neurological symptoms should provoke further CNS imaging and/or CSF analysis, depending on the clinical context of the patient and the results of additional diagnostic tests. The authors believe that when B cell lymphoma is suspected, CSF examination is the first method of choice because of its safety and high diagnostic value. Although biopsy is still the gold standard in the diagnosis of CNS lymphoma, periprocedural risk can be avoided by CSF examination. It also requires a highly skilled neurosurgical team, which is not always possible and can lead to false negative results.
Anomalies of the peripheral nervous system come about in 5% of patients with lymphoma. Some of the drugs used in lymphoma commonly cause neuropathy. Compression or infiltration of nerve roots by lymphoma is a rare presenting distinction, but becomes more common with forward disease. Either multifocal infiltration of nerves or lymphoma-associated vasculitis may present as a peripheral neuropathy. The frequency of Guillain–Barré syndrome, and possibly chronic idiopathic demyelinating polyradiculoneuropathy, appears to be enlarged in association with lymphoma. Treatment of the hidden lymphoma is only rarely followed by recovery of the associated neuropathy.28







