INTRODUCTION
This paper aims to review and redefine certain concepts of epilepsy in the light of contemporary brain research, and highlight the involvement of non-rapid eye movement (NREM) sleep homeostasis. This robust biological healing force carries a risk of epileptic upregulation; therefore, epileptogenesis is interconnected with homeostatic plasticity. The diversity of epileptic syndromes originates from the differences in brain networks and epilepsy-hosting functions; that undergo latent, long-lasting histological reorganization during periods of clinical silence. The system-epilepsy approach may provide a “royal” conceptual framework of understanding epilepsies.
The link between NREM sleep and epilepsy has attracted the attention of clinicians and neuroscientists in recent decades. In most clinical papers, authors have recognized the high prevalence of sleep disorders in the epileptic population; also calling attention to the interconnection between sleep and epilepsy: epilepsy impairs sleep-quality, and changes in sleep-physiology may activate epilepsy.1,2 The acquisition of knowledge on NREM sleep microstructure provides several examples for both effects.3 The idea that sleep physiology may be connected to epilepsy is becoming a subject of interest.4,5The recognition of homeostatic use-dependent slow-wave regulation (Figure 1)6 was an important step. Sleep researchers have recognized why NREM sleep is so important and why it is preserved throughout phylogeny, constituting about two-thirds of human life. An increased sleep-propensity after sleep deprivation shows that slow wave-power needs to be compensated. The compensatory elevation of sleep pressure resulting in slow waves, recovers synapses loaded by daytime information.7,8 However, the physiological mechanisms of this process need further clarification.
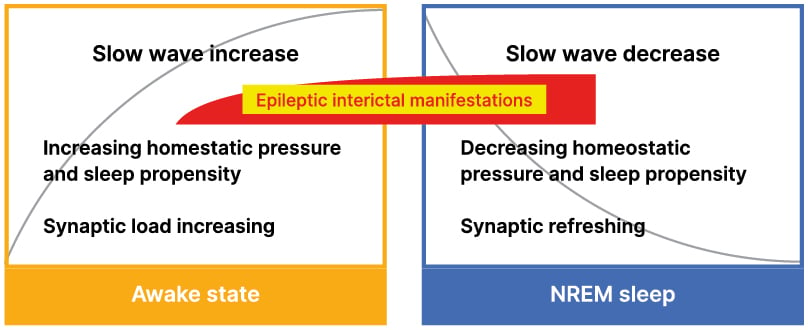
Figure 1: Dynamics of sleep pressure, synaptic load, and epileptic activity.
Homeostatic pressure, sleep propensity, slow sleep waves, and synaptic economy; changes in waking and NREM sleep. The homeostatic pressure, slow wave power and synaptic load increase exponentially in waking; reaching their highest levels during the first cycles of sleep. Later, each of them decreases exponentially with the synaptic recovery. The red/yellow insert indicates the degree and timing of interictal epileptic manifestations at the end of the day and the first sleep cycles. Yellow quadrate: waking state, blue quadrate: sleeping.
The significance of NREM sleep and its use-dependent homeostatic regulation is now better understood. NREM sleep as the main cradle of our cognitive and plastic operations has been recognised. Sleep might have been one of the powerful vehicles helping humans to stand out from the animal world.
The co-elevation of homeostatic sleep plasticity with slow wave pressure is another important point.9 The idea that epilepsy would be resulted from the exaggeration and derailment of plasticity,10,11 brings sleep and epilepsy into a close relationship.
In a cat model of postinjury epilepsy, the Timofejev school (CERVO Brain Research Centre, Université LAVAL Québec, Canada) observed a strong increase of slow wave down-states during NREM sleep; it penetrated into REM sleep and even into wakefulness.12,13 The increase of slow wave down-states paralleled an accumulation of seizures. Recently, Massimini et al.14 have observed the increase of similar sleep-like down-states in a stroke patient, during their recovery period.
THE PRESENT VIEW ABOUT THE PLAYERS OF EPILEPTIC MECHANISMS
Clinical epileptologists and researchers have an implicit agreement that epileptogenesis is the result of exaggerated synaptic excitation in certain parts of the brain, affected by a lesion or a genetic abnormality.15,16 Brain-functions are not organised in centers; rather in networks. The networks of simple brain functions are easy to locate, but tracing them in case of more complex ones is more difficult. For example, the executive memory system works in the hippocampus and is connected to cortico-frontal areas; in addition it may execute different tasks in the daytime versus during NREM sleep.
THE UPGRADING (EXAGGERATION) OF BRAIN NETWORKS IS REPRESENTED BY ELECTROPHYSIOLOGICAL MANIFESTATIONS
Since EEG has become an essential tool of epilepsy-diagnostics, the enhancement of sleep-related interictal epileptiform discharges (IED)- spikes and ripples -has been noticed, and recent ultra-long EEG observations have alighted the features of IEDs even better. Their continuous presence in epilepsies has suggested important roles, and neuroimaging studies have identified their morphological substrates.16,17 The links between IEDs and ictal events remain unclear. It is difficult to explore the direct relation between ictogenesis and the clinically silent IEDs; their participation in epileptogenesis can be considered just retrospectively in most cases. The idea about IEDs’ important role in the epileptic mechanism comes primarily from their presence in almost all types of epilepsy. In even severe epilepsies (see Electrical Status Epilepticus in Sleep [ESES]), seizures may be lacking or scarce, and IEDs be the effective players. In the major epileptic syndromes, IEDs are found close to the seizure-onset zones; typically in NREM sleep. It seems that IEDs act latently in epileptogenesis. The seizure-onset zones can be identified, but the ictogenic factors may be hidden.
Ictal triggers have been discussed recently. They can be categorized as sensory stimuli, cognitive or emotional cues,18 and sleep-related processes such as falling asleep, or arousing from sleep,19 which are special, newly considered mechanisms.
The group of IEDs has been extended to fast, pathological ripples (>200 Hz; high-frequency oscillations [HFO]) that have qualified as the most accepted biological markers of epilepsy.20 HFO cannot be detected with scalp electrodes in most EEG-labs; therefore their routine clinical use is delaying.
While the details of electrophysiological features exceed the frames of this paper, some essential phenomena deserve to be mentioned; such as the ubiquity of interictal, and (less so) the ictal spiking and the recognition of IEDs’ significance. HFO, identified recently, have been associated with cognitive processes. The extension of detected EEG oscillations to the 200–500 Hz range has revealed that HFO are valid biological markers of epilepsy20 and good tools to define the epileptogenic zone for epilepsy surgery.
The registration of HFO activity might also indicate the progress or regress of the epileptic process.
The close correlation between sleep slow waves and IEDs was described early, and has been confirmed in several papers.3,21 Later, seminal publications pointed out the transformation of sleep EEG-patterns to epileptic spikes and pathological (>500 Hz) ripples.11,22 The evident correlation of sleep slow waves with IEDs is another argument for the connection of NREM sleep with epilepsy.1 Clinically, the significance of NREM sleep as an enhancing factor is confirmed; boosting IEDs’ networks and IEDs themselves; both spikes and pathological ripples (≤500 Hz).23
NETWORK-BASED VIEW OF EPILEPSIES AND THE ROLE OF NREM SLEEP
The network-view of brain functions has appeared in the network-approach of epilepsy as well Beenhakker and Huguenard stated in their emblematic paper that “normal brain circuits provide a template that epileptic circuits use to generate seizures.”24 In other words, normal and epileptic brain circuits share common features. They demonstrated the epileptic transformations in two syndromes: medial temporal lobe epilepsy and absence epilepsy. This view automatically brought back the idea of approaching different epileptic syndromes as system (-function) epilepsies.25-28
INTERRELATIONS OF BRAIN SYSTEMS AND MAJOR EPILEPSY SYNDROMES
In the last 7 years, the authors have tried to discuss the association of well-known, frequent epileptic syndromes with established functional brain networks. Here, they provide a summary (Table 1) of this work. Out of the 10 discussed epilepsy syndromes, sleep studies are missing for reading epilepsy and human post-traumatic epilepsy, but in the rest of syndromes, a close connection with NREM sleep could be established.
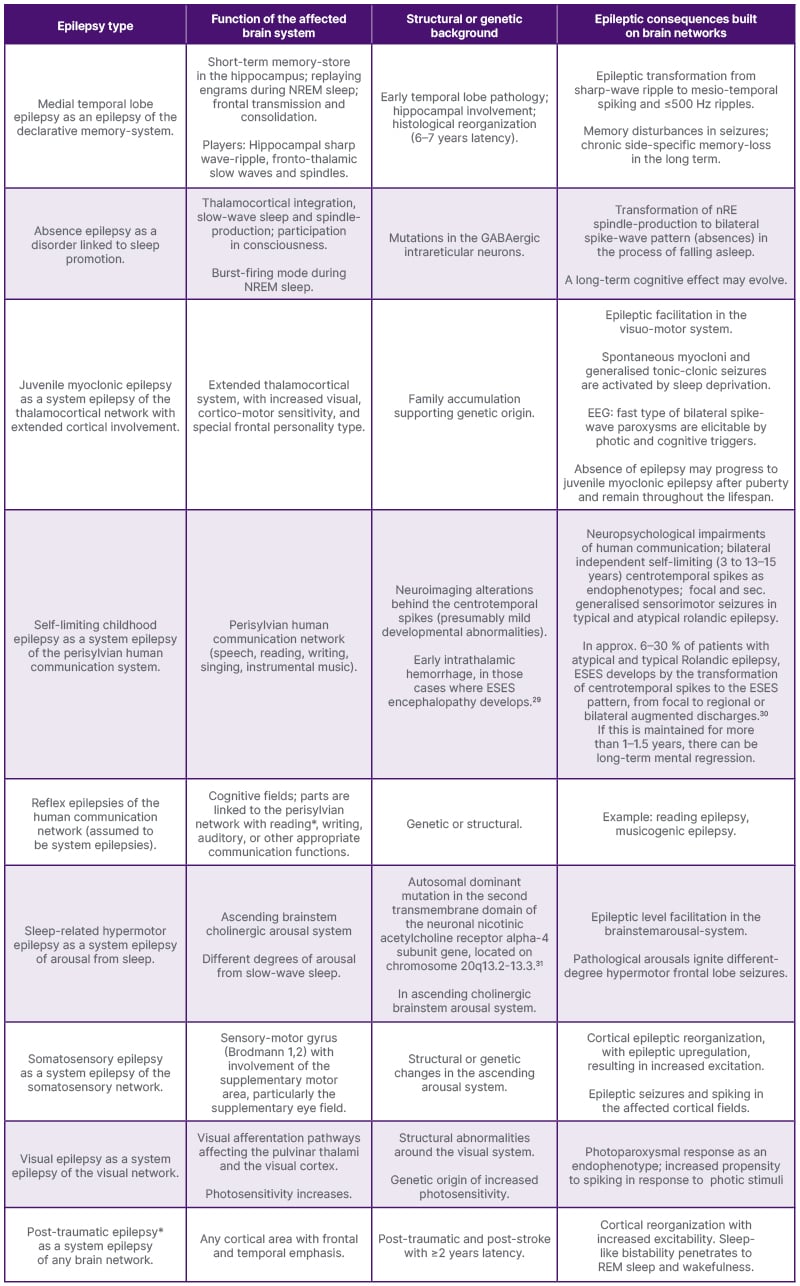
Table 1: A system-based classification of known epilepsies.
The first column shows the epilepsy type, the second describes the function of the brain system hosting epilepsy; the third one highlights etiologies; and the fourth column indicates the transformation (upgrading) to an epileptic mechanism with some consequences. However different the etiologies may be, the underlying mechanisms and the clinical and electrophysiological features seem to be common.
CHRNA4 gene: neuronal nicotinic acetylcholine receptor alpha-4 subunit gene; CTS: centro-temporal spike; EEG: electroencephalogram; ESES: Electrical Status Epilepticus in Sleep; GABA: gamma-aminobutyric acid; GTC seizure: generalised tonic–clonic seizure; HFO: high frequency oscillations; LKS: Landau-Kleffner Syndrome; nRE: reticular nucleus of the thalamus.
*For reading and post-traumatic epilepsies, sleep studies are missing.
The work of Wolf has offered much insight into the nature of ictogenesis.18 The key syndrome is juvenile myoclonic epilepsy with reflex-traits (photosensitivity, eye closure sensitivity, praxis- and language-induction). Multimodal investigations of cerebral functions indicate that ictogenesis (ab-)uses pre-existing functional brain-networks (central nervous system subsystems) normally serving physiological functions; supporting the concept of system-epilepsy.
Thus, the actual epileptic trigger is the activation of an epileptically upgraded functional network. Accordingly, absence epilepsy is perceivable as the epilepsy of NREM sleep-promotion; sleep-related hypermotor epilepsy as the epilepsy of the arousal from NREM sleep19; and self-limiting childhood epilepsies as those of the human communication network (Table 1).
Shared Features of Epileptogenesis:
- Epileptogenesis prefers those networks involved in plasticity; it favours developmental periods, and may resolve.
- The long process of epileptogenesis is ignited by a ‘‘first hit”, which is a genetic mutation or a lesion (sometimes shown by neuroimaging, e.g. in medial temporal lobe epilepsy and lesional epilepsies upon developmental anomalies).
- It carries a risk of escalation in both localization and severity, e.g. to epileptic encephalopathies (Table 1).28
- Epileptogenesis and epileptic progression are both underlain by an increase and spreading of excitation, shown by the shared presence of pathological HFO.32–34
- NREM sleep enhances IEDs, promoting epileptogenesis. Sleep oscillations become templates for IEDs, the clearest example being the transformation of hippocampal sharp wave-ripple to epileptic spike-pathological ripple.11,22
- IEDs have crucial role in epileptogenesis. They have specific distributions in relation with the up- and down-states of sleep slow waves.1 Sleep spindles link to the “up” states, and HFO predominantly occur in the transition-zones from the “up” to the “down” state. These data highlight the so-called ‘‘bistability” (or alternating polarity) of sleep slow waves as a potential factor driving epileptogenesis in sleep.
INTERRELATIONS OF BRAIN SYSTEMS AND MAJOR EPILEPSIES
Epilepsy is a colourful ensemble of syndromes; a few of them are frequent, while the large majority appear rarely.
The authors are focusing here on those epilepsies frequently encountered by neurologists (Table 1).
For dealing with system epilepsies, one needs to be familiar with the affected brain systems’ neuroanatomy. The criteria of system epilepsies are simple: seizure symptoms reflect, by an excitatory or inhibitory way, the function of the system; the localization of epileptic EEG-changes covers the anatomy of the system; if there is a lesion, it is in anatomical relation with the epilepsy-network; a long-term dysfunction may evolve in the affected system(s); and if a reflex seizure-trigger can be identified, it is congruent with the afferentation of the system.
The diversity of epilepsy-presentations does not contradict the existence of a common way of epileptogenesis. The multifariousness of epilepsies is related to the diversity of involved brain-networks.
SYSTEM EPILEPSY AND EPILEPSY TAXONOMY
The classification of epilepsies needs to reflect contemporary perception. The International League Against Epilepsy (ILAE) taxonomy is struggling to maintain the focal/generalized dichotomy, despite convincing evidence that “focal” epilepsies are not circumscribed enough and “generalized” epilepsies are insufficiently generalized.35 The network concept now prevailing in brain research may resolve this contradiction and the untenable separation of epilepsy-research from the mainstream of network-based approaches in neuroscience.
The system-view of epilepsy raises several questions; nonetheless it seems to provide a uniform comprehensive approach with flexible frames.







