Abstract
Additional efficacious treatment options for stroke are urgently needed as thrombolysis and endovascular thrombectomy are too rarely utilised due to the narrow time window. This article reviews the clinical profile of Cerebrolysin® (EVER Pharma, Austria), a well-documented compound indicated for the treatment of acute stroke, which has also shown promising results in neurorecovery. Well-conducted clinical trials have shown strong and encouraging treatment signals, either as a single therapy or in combination with recombinant tissue plasminogen activator. This review focusses on the latest research results, especially on the randomised, controlled CARS study, which combined pharmacological and rehabilitation therapy. Furthermore, this study reached the primary endpoint at Day 90 in the Action Research Arm Test and showed significant improvements in modified Rankin Scale (mRS) at Day 90. The same applies to important secondary endpoints such as the National Institutes of Health Stroke Scale (NIHSS) at Day 21 (early benefit). CARS is a rehabilitation study with a larger sample size and gives neurologists as well as rehabilitation specialists renewed confidence in this class of neuroprotective/ neurorecovery-enhancing compounds. Furthermore, ongoing research projects like CREGS-S are discussed, a large-scale prospective registry that adds valuable real-world data to the existing portfolio of Cerebrolysin studies.
Stroke poses a major health problem today, both medically and socially. Worldwide, ~15 million people suffer from stroke each year and 5 million die thereof within a year. Stroke is the third most common cause of death following cardiovascular diseases and cancers.1 More than 35 million patients live with stroke sequelae and many require substantial help. In 2008, indirect and direct costs associated with stroke were estimated at $65 billion in the USA alone, with a significant part thereof related to post-stroke care.2
Over the last decades, therapeutic approaches for stroke have significantly evolved and improved. This is a natural consequence of the implementation of modern stroke units, improvement of general medical care, and more structured and early administered rehabilitation schemes. Thrombolytic therapy with recombinant tissue plasminogen activator (rtPA) has been spread, and a number of clinical trials have recently confirmed the effectiveness of thrombectomy to be higher than that of rtPA alone.3 However, the main downfall of rtPA use is its narrow therapeutic time window (3.0–4.5 hours after the onset of symptoms), which limits its use to 10–20% of all stroke patients.4 Similarly, the use of thrombectomy, hindered by operational reasons, is relatively rarely performed now and even in the future might not encompass more than a few percent of patients.3
Except thrombolytic therapy and mechanical removal of thromboembolic material, there is still no widely accepted therapy for acute ischaemic stroke. Statistical data show that even if advanced procedures can be used, 60% of stroke patients die or remain handicapped.1 Since immobilisationrelated complications cause >50% of stroke patients’ deaths, rehabilitation plays a vital part in stroke care.5 According to recommendations of the European Stroke Organisation (ESO), poststroke active rehabilitation should be initiated as early as possible and aim to regain lost abilities of pre-stroke functions, adapt to post-stroke life, and establish an optimal level of independence.6
Continuous research has been carried out to develop medications with modulating properties that facilitate natural brain plasticity and provide a comprehensive therapy after both ischaemic and haemorrhagic stroke.7 In a holistic therapeutic approach, a combination of pharmacological treatment and rehabilitation methods may have additive or even synergistic effects in the process of brain recovery.8
One such medication that can be used in auxiliary stroke treatment is Cerebrolysin. Cerebrolysin contains a mixture of neuropeptides and free amino acids with pharmacologic properties similar to those of endogenous neurotrophic factors. Preclinical studies in animal models of acute brain injury showed the pleiotropic neuroprotective and recovery-promoting effects of Cerebrolysin by interfering with different steps of the pathological cascade and modulating endogenous defence activity.9-14 This multimodal action of Cerebrolysin has been reviewed previously.15
Former clinical stroke studies had small patient numbers and differed in clinical outcome criteria, as well as therapy onset and duration.16-18 Nevertheless, all trials demonstrated a benign safety profile of Cerebrolysin and beneficial clinical effects. As a consequence of the positive findings from clinical studies, Cerebrolysin is nowadays routinely used in stroke centres around the world. The American Stroke Association’s (ASA) guidelines from 2013 acknowledged a study with Cerebrolysin that deemed it safe and showed trends towards favourable clinical outcomes.19
The results of recently published large and well-controlled clinical studies show a distinct and potentially positive effect of Cerebrolysin on neurological recovery from acute ischaemic stroke in selected patient groups. Among these, the CASTA trial, published in 2012 with 1,070 patients, demonstrated interesting results.20 The primary study results were neutral in the total patient population; however, it is important to consider the mild stroke pathology at baseline that caused ceiling effects at Day 90 (median National Institutes of Health Stroke Scale [NIHSS] of 9 in both groups). However, a post hoc subgroup analysis in patients with more severe stroke (NIHSS >12 points) showed a significantly higher improvement than the placebo group as evidenced by the NIHSS score at Day 90. Of note, this was accompanied by a significant reduction in the cumulative mortality rate of patients treated with Cerebrolysin (10.5%) compared to placebo (20.2%), see Figure 1. The study furthermore confirmed the excellent safety profile of Cerebrolysin with no apparent differences in any of the safety parameters between Cerebrolysin and placebo.
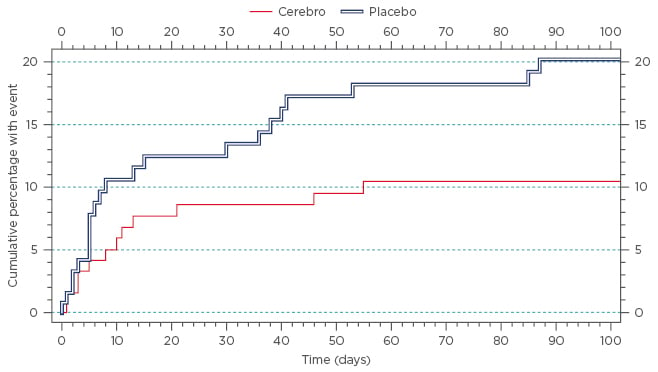
Figure 1: Kaplan-Meier survival curve (cumulative percentage) for subgroup baseline NIHSS >12 points (N=252, 126 patients per group); ITT population.
Hazard ratio: 1.9661, confidence interval lower bound: 1.0013.
ITT: intention-to-treat; NIHSS: National Institutes of Health Stroke Scale.
Taken from Heiss et al.20
In 2013 Lang et al.21 published the results of a randomised, placebo-controlled Cerebrolysin study in thrombolysed patients. The 10-day treatment with Cerebrolysin did not show a significant group difference Day 90 in the primary parameter (modified Rankin Scale [mRS]). However, the number of patients with an improvement in the NIHSS of ≥6 points was significantly higher in the Cerebrolysin group as compared to placebo at Days 2, 5, 10, and 30 post-stroke (Figure 2). The use of Cerebrolysin in combination with rtPA was safe with no differences in any of the safety parameters.
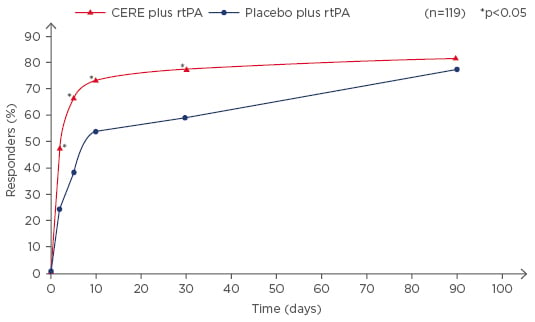
Figure 2: Responder analysis (improvement of at least 6 points from baseline or total score of 0–1; ITT population) of Cerebrolysin (n=60; 30 ml/10 days; treatment start immediately after rtPA) and placebo-treated (n=59) patients. Proportion of responders in NIHSS at baseline and Days 5, 10, 30, and 90. N=119; *p<0.05 versus placebo).
CERE: Cerebrolysin; rtPA: recombinant tissue plasminogen activator; NIHSS: National Institutes of Health Stroke Scale.
Taken from Lang et al.21
The recently published CARS study assessed the efficacy and safety of Cerebrolysin in combination with a standardised rehabilitation programme. The primary study endpoint was the Action Research Arm Test (ARAT) at Day 90, assessing upper-limb motor function. Cerebrolysin was administered for 21 days, starting within 48–72 hours after ischaemic stroke.22
The CARS study showed not only a statistically significant group difference in the primary study endpoint, but Cerebrolysin was also observed to be superior over placebo in most of the secondary endpoints like the NIHSS, Barthel Index, and mRS (Figure 3). Moreover, at Day 90, patients treated with Cerebrolysin showed fewer depressive symptoms and an improved quality of life. In addition, the most important measure for early benefit, the NIHSS at Day 21, showed a significant superiority for Cerebrolysin. Analysis of the safety parameters, such as adverse events, serious adverse events, vital signs, and laboratory parameters did not show any clinically important differences between the treatment groups. The trial indicates that the early combination of rehabilitation with a multimodal medication of neuroprotective and plasticity-supporting properties is a valid therapeutic approach.
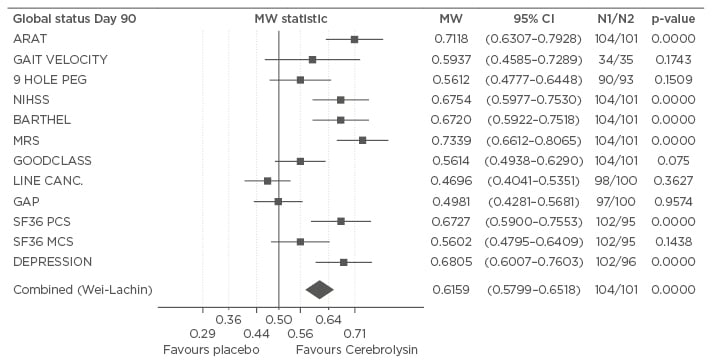
Figure 3: Global status on Day 90. The effect sizes (MW) for the single and combined (Wei–Lachin procedure) efficacy parameters reflect changes from baseline in the modified intention-to-treat last observation carried forward population (n=205).
Analyses were conducted using the multivariate, directional Wilcoxon test.
MCS: mental component summary; mRS: modified Rankin Scale; PCS: physical component summary; MW: Mann–Whitney; CI: confidence interval; ARAT: action research arm test; NIHSS: National Institutes of Health Stroke Scale.
Taken from Muresanu et al.22
Although CARS, CASTA, and the study by Lang et al.21 had individual designs, obtained data suggest most promising treatment effects of Cerebrolysin in patients with moderate or severe stroke and treatment initiation early after stroke.
A new ongoing research project is the prospective Cerebrolysin Registry Study in Stroke (CREGS-S). Registries are a very valuable tool to assess drug effectiveness and safety in daily clinical practice and may in some cases substitute clinical trials.23 The protocol has been designed to collect data in a standardised manner, including stroke severity, treatment schemes, concomitant diseases, and medication. Also, early and late treatment effectiveness is documented by using the NIHSS and mRS (ClinicalTrials.gov Identifier: NCT02541227). The CREGS-S registry and the recent studies will help to optimise further treatment with Cerebrolysin to ensure the highest benefit for the stroke patient.








