Abstract
Non-dystrophic myotonias (NDM) manifest as delayed muscle relaxation leading to muscle stiffness. This may diminish or worsen with repeated contractions, depending on NDM subtype. These are divided into those affecting the chloride channel CLC-1, due to CLCN1 gene mutations, and those affecting the sodium channel NaV1.4, due to SCN4A gene mutations. Depending on NDM subtype, additional symptoms and clinical signs of NDM can include transient weakness, myalgia, cramps, fatigue, dysphagia, dysphonia, and muscle hypertrophy. Two surveys, carried out independently but collectively named IMPACT (Impact of non-dystrophic Myotonia on PAtients and Caregivers’ qualiTy of life), were conducted to help elucidate how symptoms affect adults with NDM and those who care for adults or children with this condition. The patient survey not only confirmed NDM symptoms experienced by participants, but also highlighted how such symptoms affect a person’s quality of life, mental health, and abilities including problems with work, study, childcare, and socialising. Additionally, details of the diagnostic pathway, treatment, and healthcare professionals involved in NDM were revealed. The caregiver survey found that almost one-third of those who cared for someone with NDM did so for at least 10 hours per week. It also highlighted how a carer’s physical and mental health could be impacted by caregiving, potentially due to the finding that half of respondents felt that they had little or no support. Presented here are highlights of the IMPACT survey along with insights from five NDM clinical experts: Jordi Diaz-Manera, Channa Hewamadduma, Giovanni Meola, Federica Montagnese, and Sabrina Sacconi.
INTRODUCTION
Non-dystrophic myotonias (NDM) manifest as delayed muscle relaxation (myotonia) leading to muscle stiffness that may diminish or worsen with repeated contractions, depending on NDM subtype.1 Symptoms and clinical signs, also dependent on subtype, can include transient weakness, myalgia, cramps, fatigue, dysphagia, dysphonia, and muscle hypertrophy.2,3
NDM arise due to skeletal voltage-gated muscle channel defects caused by mutations in CLCN1 or SCN4A genes.3CLCN1 codes the chloride channel CLC-1, so related conditions are classified as ‘chloride channelopathies’; CLCN1 mutations lead to Thomsen myotonia congenita (TMC;4 in an autosomal dominant pattern of inheritance) or to Becker myotonia congenita (BMC;5 in an autosomal recessive pattern of inheritance). SCN4A mutations, also inherited in an autosomal dominant manner, code the NaV1.4 channel, with related conditions classified as ‘sodium channelopathies’; these NDM include paramyotonia congenita (PMC),6 sodium channel myotonia, and hyperkalaemic periodic paralysis.3
Considering that NDM is a rare condition, with point prevalence estimates ranging from 0.75 to 1.70 per 100,000 people,7,8 it is unlikely that many healthcare professionals (HCPs) will diagnose a patient with NDM throughout their clinical career.
IMPACT SURVEY
While descriptions of NDM symptoms and signs can be found easily,3 the impact of NDM on daily life is not clearly established. A thorough analysis of this could lead to better understanding of NDM, clearer ideas about treatment, and design of scales to better monitor symptoms and treatment response.
With this in mind, between August and October 2020, market research in the form of an online survey among adults (≥18 years) with NDM and another among carers of people with NDM, collectively named IMPACT (Impact of non-dystrophic Myotonia on PAtients and Caregivers’ qualiTy of life), was conducted by admedicum®, Cologne, Germany, partnering with Lupin Neurosciences, Zug, Switzerland. The surveys were reviewed by patient experts prior to finalisation and were available in English, Dutch, French, Italian, Spanish, and German. Methods and preliminary results were presented at the 16th International Congress on Neuromuscular Diseases, 2021.9
The surveys questioned respondents on diagnosis, prior and current symptoms, abilities affected, impact on quality of life (QoL), treatment, and support. Both surveys were anonymous and confidential, with declaration of NDM/carer status completed by the respondent only. No data were collected that would permit participant identification. Respondents were recruited via patient organisations, HCPs, and social media promoted through Google advertisments.
The patient survey was completed by 181 people from 27, mostly European, countries. The highest numbers of respondents were from the USA (n=39), Germany (n=35), the UK (n=32), the Netherlands (n=15), and Spain (n=10). Respondents were predominantly female (62%) and between 31 and 65 years of age (71%), with 24% aged 18−30 years.
The caregiver survey included self-identified, non-professional caregivers of both minors (29%) and adults (71%). A total of 59 caregivers from 12 countries responded, with most from the UK (n=18), Germany (n=14), USA (n=7), Spain (n=4), Italy (n=4), and Sweden (n=4). Most respondents were female (75%), the patient’s parent (42%) or partner (39%), and aged between 18 and 45 years (65%) or 46 and 65 years (32%).
At a roundtable meeting, five clinical experts, Jordi Diaz-Manera, Channa Hewamadduma, Giovanni Meola, Federica Montagnese, and Sabrina Sacconi, discussed NDM symptoms, diagnosis, treatment, and outcomes from the IMPACT surveys. Their views on the IMPACT survey results and their clinical expertise is presented here.
DIAGNOSIS
NDM diagnosis starts with ascertaining age of onset, family history, symptoms, and exacerbating factors, such as exercise, cold, menstruation, hunger, dietary potassium, psychological stress, and alcohol.2,3 Key initial diagnostic steps are neurological examinations and ‘bedside’ tests of motor function, including observations of stiffness level when initiating movement and muscle relaxation after a contraction.3
As a consultation is only a ‘spot check’ of symptoms because of their unpredictability, Meola recommended patients fill out a symptom diary to help reveal exacerbating factors. This could be a paper diary or an interactive voice response system to record daily symptom severity and frequency.10
Follow-Up Tests
Electromyography can reveal electrical myotonia as distinct Fournier patterns of self-sustained bursts of discharge after a short exercise test (sometimes with cooling).11 While this can guide towards NDM categorisation, there are crossovers between the genetically distinct NDM subtypes.11,12
Genetic testing can help confirm NDM subtype3 and with rapid sequencing the panel reported how wait-time for such confirmation should be only a few weeks. However, in the IMPACT survey, 19.3% of respondents had not had their diagnosis confirmed genetically.
Diagnostic Delays
The IMPACT patient survey revealed that 65% of respondents experienced symptoms for more than 10 years prior to diagnosis; for others this was 5−10 (15%) or 2−5 (12%) years. According to Hewamadduma, one reason for diagnostic delay is that younger patients may be told that symptoms are just a normal part of growing up so “they put up with [them] until maybe […] at a sports event when the pistol goes, they can’t start running.”
According to Hewamadduma, once a patient presents to their general practitioner (GP), there may be a delay as “the GP may not have encountered a patient with myotonia before.” Diaz-Manera agreed, saying that “sometimes [symptoms are] tightness, for others it’s weakness or many other complaints that are not specific, so…for GPs it’s difficult to think about congenital myotonia as an option.”
Further delay may occur if a patient is not sent for appropriate specialist care. For example, instead of being referred to a neuromuscular specialist, patients might see a rheumatologist, orthopaedist, or general neurologist. While the latter may seem suitable, Diaz-Manera highlighted how even general neurologists are sometimes not familiar with NDM. This is compounded, according to Montagnese, by NDM-specific examinations not being part of a common neurological training or education. Encouragingly, Sacconi reported that in her centre, educating general neurologists to include NDM-focused electromyography has led to increased referrals of patients with NDM to neuromuscular specialists.
SYMPTOMS
Of the 181 IMPACT respondents, 28.2% had TMC, 25.4% had BMC, 17.1% had PMC, 4.4% had ‘potassium-aggravating myotonia’, and 2.2% had ‘periodic paralysis’. The remainder reported undefined ‘myotonia congenita’ (11.0%), a general/other NDM diagnosis (5.0%), or were awaiting classification (6.6%).
NDM subtypes can be somewhat distinguished by age of onset and symptoms. Chloride channelopathies usually start at approximately 10 years of age.3,13,14 Although some BMC and TMC symptoms are similar, such as myotonia decreasing after a ‘warm-up’ period, others, such as muscle pain and hypertrophy, transient weakness, and upper limb involvement, are more likely in BMC, while lower limbs are predominantly affected in TMC.
Sodium channelopathies tend to start at approximately 5 years of age with symptoms including facial stiffness (often involving eyelid myotonia), pain, and episodic weakness, all exacerbated in cold weather.3 In PMC especially, myotonia may increase after repetitive contractions, while for some with sodium channel myotonia, potassium consumption can aggravate the symptoms.3
Although some symptoms may differ by diagnosis, nearly all IMPACT respondents reported experiencing many of the core symptoms, such as limb muscle stiffness and mobility problems, on a daily or continuous basis and experiencing other symptoms, such as muscle pain and headache, at least sometimes (Table 1). Other symptoms reported at least sometimes were gastrointestinal issues (69%), being unable to open their eyes after sneezing/blinking (64%), a risk of dropping drinks (62%), and their throat closing up when consuming cold drinks or food (45%).
Participants could also provide free-text comments about how symptoms affected them:
- “Crossing the street is a big problem.”
- “Eating bananas, even a small piece, makes my body stiff.”
- “Inability to concentrate, especially in the mornings after I have exercised the day before.”
- “Constant feeling of exhaustion. More than normal pain and longer restitution time needed after exercising.”
QUALITY OF LIFE
NDM symptoms can greatly impact a person’s QoL; while 48% of IMPACT patient respondents rated their QoL as high (at least 7 on a scale from 0 to 10 [worst to best possible condition]), 27% rated it as low (0 to 4). Many also reported constant worry due to symptom unpredictability and about NDM progression (Table 1).
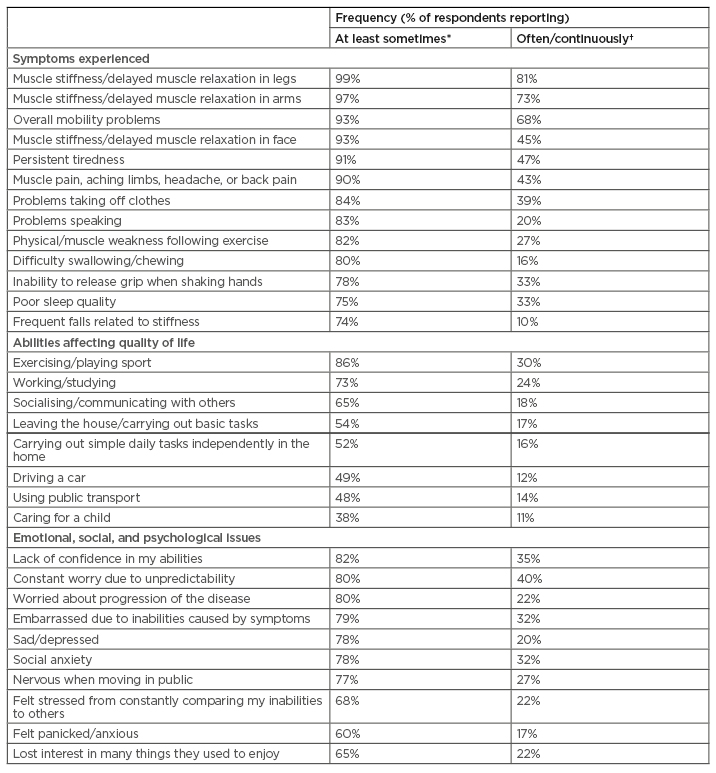
Table 1: Symptoms experienced, quality of life factors, and emotional, social, and psychological issues listed by respondents when asked “Within the last 6 months, how often have you experienced the following symptoms?” (N=181).
*Sometimes: a few times per year or month; regularly: weekly; often: usually daily; continuously: several times per day. †Often: usually daily; continuously: several times per day.
IMPACT results revealed that areas of life affected included exercising/playing sport, working/studying, and socialising/communicating with others (Table 1), as well as attending classes (36%) or finding a partner (19%). Understanding and addressing factors beyond physical symptoms could help improve the QoL for a person with NDM.15 According to Hewamadduma, the symptoms that most interfere with QoL depend on a person’s specific circumstances. For example, one patient, a cricket player, had few problems practising in the summer but struggled in the winter cold. For another, their problem was being unable to walk their child to school and for a third, throat spasms when eating anything cold were problematic. Montagnese also pointed out how pain is another fundamental complaint impairing QoL.
NDM effects also include major life events. Over half (52%) of 122 participants said that NDM had restricted their abilities, indicating that it had also negatively impacted their education or career at least once. Over a quarter felt unable to apply for their preferred education/job (26%) and some had to terminate/lose a job (19%) or were not able to carry out their trained profession (17%).
Patients also expressed how NDM affected them psychologically (Table 1); for example, many felt sad or depressed, lacked confidence in their abilities, experienced social anxiety, or were embarrassed due to their limiting symptoms. Some revealed that having NDM made them feel isolated (57%), feel alone and helpless (49%), and cry a lot (49%). Over a third (39%) reported that they had been bullied due to NDM.
Respondents provided free-text comments regarding how NDM symptoms affected them psychologically/socially:
“Fear of open spaces…severe anxiety, depression.”
“Discrimination when I go to the gym because I can’t do everything…and can’t tolerate bright lights and music.”
“Explaining to people who did not realise your limitations can be embarrassing.”
“Pain and fatigue have the most effect on mental state. Not having energy enough to do things others do…always having to make concessions.”
To help with this aspect of NDM, Hewamadduma discussed how well-structured mental health support is essential and Meola emphasised the importance of encouraging patient networking groups, especially as there is no formal patient association for NDM.
TREATMENT
The survey results found that only 23% of respondents were ‘satisfied’ or ‘very satisfied’ with current symptom management, with 33% ‘moderately satisfied’ and 34% ‘not satisfied.’ While 29% of respondents reported they were not receiving or had never received NDM-specific treatment, 59% were currently taking/had taken a medication for NDM, 33% received physiotherapy, and 16% received psychological support.
There is currently no disease-modifying treatment for NDM; however, the Class IB antiarrhythmic mexiletine has European Medicines Agency (EMA) approval as an orphan drug to treat myotonia symptoms in adults with NDM. Mexiletine reduces or abolishes muscle hyperexcitability for both chloride and sodium ion channelopathies by inducing a slower sodium influx, thus enhancing fast inactivation and faster repolarisation of sodium channels.3 Randomised, placebo-controlled and n-of-1 clinical trials have shown mexiletine’s effectiveness for reducing muscle stiffness, weakness, and pain and in improving QoL in NDM.10,16 Treatment requires cardiac monitoring prior to or during mexiletine as it may exacerbate pre-existing arrhythmia.17 Other off-label treatments include lamotrigine (the only other drug with a placebo-controlled trial in NDM18), carbamazepine, flecainide, acetazolamide, and ranolazine.3
Of the 89 IMPACT respondents currently taking a medication, mexiletine was the most commonly prescribed (n=45), followed by lamotrigine (n=10), flecainide (n=9), carbamazepine (n=9), acetazolamide (n=8), quinidine sulphate (n=7), calcium antagonists (n=6), phenytoin (n=2), baclofen (n=1), magnesium (n=1), or propafenone (n=1).
Figure 1 shows how often the most-experienced NDM symptoms occurred: the higher the number, the more often a symptom was experienced (1=never; 5=continuously). These were also reported as the symptoms patients most wanted improved by treatment. Also shown in Figure 1 is how much these symptoms were generally improved by drug treatment for those respondents receiving treatment: the higher the number, the more significant a symptom was improved (1=did not improve at all; 5=significant improvement).
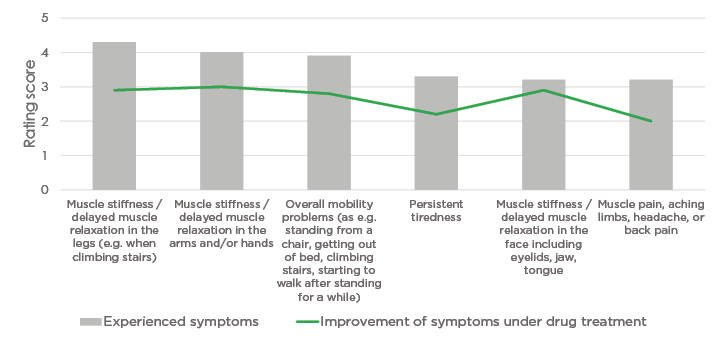
Figure 1: Correlation between frequency of experienced symptoms and improvement of symptoms under drug treatment for the top six symptoms experienced.
Mean for experienced symptoms: the higher the number, the more often a symptom was experienced (1=never; 5=continuously). Mean for improvement of symptoms: the higher the number, the more significantly a symptom was improved (1=did not improve at all; 5=significant improvement).
Free-text responses regarding drug treatment included positive and negative comments. Comments were separated from other responses and aggregated so cannot be tied to a particular medication:
- “Through medication my QoL has increased. I can participate in more things or preserve longer.”
- “In terms of my ability to communicate via eye contact, facial impressions, and gestures, my QoL has improved significantly.”
- “It has definitely improved self-belief/self-confidence that I can walk across a parking lot without getting stiff halfway across.”
- “Overall speaking, minimal improvement of symptoms.”
- “The symptoms of myotonia were hardly attenuated and other symptoms appeared that made my life quite complicated.”
Patients with NDM also wanted treatment to address QoL factors and reported how their ability to carry out tasks, including leaving the house, driving, exercising, working/studying, socialising, and using public transport, was improved with drug treatment. Of note, however, the average improvement of QoL symptoms, which was also on the 1−5 scale above, was between 2−3, indicating an unmet need for more comprehensive treatment in NDM to enhance their QoL.
Targeting Treatment to Symptoms Experienced
The panel agreed that NDM treatment must consider individual needs and have a holistic understanding of each patient’s behaviours, habits, and comorbidities. According to Sacconi, it is essential that the HCP and patient set treatment goals together, with Montagnese highlighting that it is vital to discuss that while the drugs are effective for some symptoms, they do not cure NDM.
Although some symptoms can be alleviated with drug treatment, Hewamadduma emphasised how in his experience, pain and tiredness are the hardest to manage: “[Patients] need a lot of counselling and goal-setting conversations, particularly those who have increased tiredness; we teach them about pacing, so [the treatment is] not only the drug itself.” “Pain is something that we are not paying enough attention to,” confirmed Diaz-Manera. That most patients are seeking pain relief was, he suggested, reflected in the practitioners that patients with NDM reported visiting, including an acupuncturist, kinesiologist, osteopath, and herbalist.
Addressing Those Not Receiving Drug Therapy
While drug treatment may help, 74 of the 181 NDM respondents reported they had not taken any NDM-directed medication. For some, this was simply because they did not want to take any medication (27%) or felt their symptoms were managed well enough without (16%). Indeed, according to Montagnese: “if we think the patient is not hugely affected in everyday life, if they are concerned about the drug and we ourselves are not sure they need a medication, it’s right not to prescribe anything.”
However, a major reason these respondents gave for not trying a drug treatment was fear of side effects (49%). “Of course, any drug has some side effects,” discussed Meola, “but we need to make the right titration and spend time to educate the patient.” A diary could be useful here, according to Sacconi, “to understand the best doses for therapy, [so as] not to have an adverse event.”
Also reported was that some did not receive treatment because their doctor did not prescribe any (28%), or they (8%) or their doctor (9%) were not aware of NDM treatment. Indeed, the big problem, confirmed Meola, is that some neurologists do not know enough about NDM myotonia treatments to feel confident prescribing them.
Patients also, according to Sacconi, enquire about an NDM-specific diet. While no targeted approach currently exists, Meola stressed the important role of the dietitian in his practice for helping some patients to understand how myotonia could be influenced by potassium.
CAREGIVER SURVEY
While surveys of people who care for someone with other muscle-related disorders, such as muscular dystrophy, highlight how caregivers can experience considerable burden,19 to date, no such survey has been carried out regarding NDM caregivers. This was addressed by the caregivers’ component of the IMPACT survey.
The diagnosis of the caregiver’s charge was predominantly myotonia congenita (BMC: 37%; TMC: 12%; non-defined: 20%), followed by PMC (8%), potassium-aggravating myotonia (5%), or periodic paralysis (2%). Many reported being a caregiver for >20 years (12%), 10−20 years (37%), or 5−10 years (31%). Almost half (45%) spent ≥5 hours per week caring, with 29% spending ≥10 hours per week. Looking at how NDM subtype may affect care, most who cared for someone with BMC reported spending <6 hours per week caregiving (19 of 22 caregivers), while four of seven caring for someone with TMC and four of five with PMC spent ≥10 hours per week providing care.
Though many caregiving respondents reported a high QoL (76% rated 7 or higher on a 0−10 point, worst to best scale), some thought that their mental health (42%) and their physical health (25%) had worsened due to caregiving, with burden linked to time spent on care. Many reported that they were ‘happy’ to care for the person with NDM and ‘felt fulfilment’ from their caregiving tasks ‘frequently’ (69% and 36%, respectively) or ‘often’ (19% and 22%, respectively); however, others reported that they were ‘not at all’ (2% and 10%) or ‘only sometimes’ (3% and 19%) happy to be a caregiver or felt fulfilment from their tasks, respectively.
Additionally, many caregivers reported that they were worried about NDM progression, were concerned about the future, and were worried about their own health at least sometimes (88%, 86%, and 61%, respectively) or permanently (19%, 14%, and 0%, respectively). Carers also, at least sometimes, reported being overwhelmed by caring (61%), having inadequate sleep (54%), feeling isolated (41%), or feeling nervous (59%).
Free-text comments from caregivers were also provided:
- “I have a complete sense of helplessness as I don’t know how to ease their pain or discomfort, and a sadness that they have lost out in many ways by not being able to participate in sports, drama, and physical activities.”
- “All the therapies, the appointments, it takes a massive toll on the whole family not just the carer or the person affected, siblings suffer too.”
- “Child has times where she is severely affected…so needs a lot of time and help with massage, getting dressed, moving up and down stairs.”
Support is something that carers need as well as patients. While 51% ‘regularly’, ‘often’, or ‘permanently’ ‘felt strongly supported by others’, 27% said this was only ‘sometimes’ and 22% said ‘not at all’. Discussing one case, Hewamadduma recounted how the mother of a child with NDM needed help because of the psychological impact of her caregiver burden. “This was not a couple of hours, this was days of psychological input as she was suicidal at one point.”
A further free-text comment was provided:
- “A big challenge/worry is very limited support from medical professionals who have little to no experience with NDM. As I don’t know if/how it will progress, it’s worrying that they won’t be able to provide me with treatment options and advice I could trust.”
Caregiver burden was also reflected in the effect on carer’s abilities. For example, approximately half reported that caregiving duties affected their ability to work, exercise, or pursue a hobby, meaning they had to, at least sometimes, change plans or combine caregiving with their own activities. A few reported that caregiving negatively impacted their education/career choices (six parents, one partner), that they had to reduce their working hours (five parents, one partner), or were no longer able to work (four parents, one partner).
Similar to those with NDM, caregivers reported that improvement of NDM symptoms of the person they cared for would, at least moderately, help reduce their caregiving burden (Figure 2).
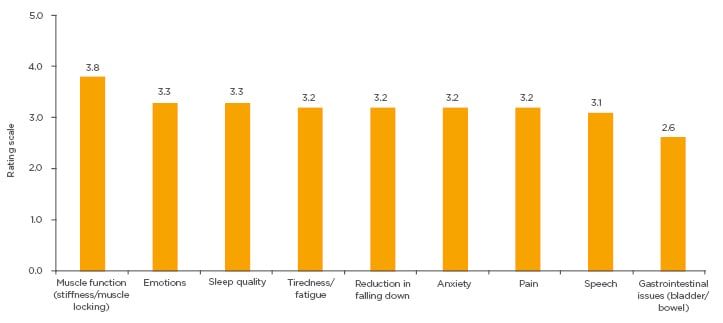
Figure 2: Results from caregiver respondents when asked: “Please rate how an improvement of symptoms of the person you care for helps (or would help) to reduce the need for your care?” (multiple choices possible).
Rating scale: 1=not helpful at all; 5=significant improvement of quality of life.
CONCLUSION
The IMPACT surveys for the first-time shed light on the extent of NDM burden for people living with the disease and those caring for them. Currently, there are no formal guidelines or consensus recommendations for NDM; the IMPACT survey results could help HCPs understand unmet needs in terms of diagnosis, treatment, and support for people with NDM and their caregivers.
The results suggest that wider access to genetic testing and earlier referral to specialised neuromuscular centres is needed, which can lead to more targeted therapy according to aetiology. There are needs to develop a globally standardised NDM symptom diary; to work with patients and HCPs to create easily accessible, reliable information about NDM and treatment options; and to develop NDM patient-to-patient exchanges so that they can find support and educate each other.
Opening the survey to more people with NDM and their caregivers could add to the current findings but more data are also needed, including international co-operation and multicentric natural history studies.








