Chairperson: Ian Kremer1
Speakers:Marwan Sabbagh,2 Carla Abdelnour,3 Claire Paquet4
1. Leaders Engaged on Alzheimer’s Disease (LEAD) Coalition, Washington, D.C., USA
2. Cleveland Clinic Lou Ruvo Center, Las Vegas, Nevada, USA
3. Fundació ACE, Barcelona, Spain
4. Center of Cognitive Neurology, University of Paris, Paris, France
Disclosure: Mr Kremer has declared no conflicts of interest. Dr Sabbagh has received royalties from HarperCollins; owns stock in uMETHOD Health, Brain Health, Inc., Optimal Cognitive Health Company, M3 Biotechnology, and Versanum, Inc.; been a member of the speakers bureau for PeerView; and served as a consultant for Allergan, Biogen, Bracket, Neurotrope, Cortexyme, F. Hoffmann–La Roche, Grifols, Sanofi, and Regeneron. Dr Abdelnour has received lecture fees from Zambon, F. Hoffmann–La Roche, and Schwabe Farma Ibérica S.A.U. Prof Paquet has served on the advisory board for F. Hoffmann–La Roche, Lilly, and Biogen; served as a consultant for Fujirebio, Alzohis, Neuroimmune, AgenT, and Gilead Sciences, Inc.; and served as an investigator in several Alzheimer’s disease clinical trials for F. Hoffmann–La Roche, Eisai, Lilly, Biogen, AstraZeneca, Lundbeck, Neuroimmune, and Green Valley.
Acknowledgements: Writing assistance was provided by Dr Eleanor Roberts, Beeline Science Communications, Ltd, London, UK.
Support: The publication of this article was funded by F. Hoffmann–La Roche. The views and opinions expressed are those of the presenters. The content was reviewed by F. Hoffmann–La Roche and the speakers for medical accuracy.
Citation: EMJ Neurol. 2020;8[Suppl 1]:2-9.
Meeting Summary
Globally, one person develops dementia every 3 seconds. While currently around 50 million people are living with Alzheimer’s disease (AD), estimates suggest this could increase to over 150 million by the middle of the century.1 Although there is no cure for AD yet, there are modifiable risk factors that, if addressed, could delay the onset and progression, or even prevent AD development.
This series of four talks was aimed at providing healthcare professionals (HCP) with an understanding of the need for early diagnosis of AD to enable refined care, with the goal of providing individuals with AD the best possible quality of life (QoL) at all stages of their condition. This will enable healthcare systems to prepare for the potential increased wave of individuals with AD set to come.
During the 2020 Alzheimer’s Association International Conference (AAIC), Mr Kremer discussed “The Secret – Speaking the Unspoken in AD,” Dr Sabbagh presented “Don’t Defy the Diagnosis, Try to Defy the Verdict,” Dr Abdelnour discussed “Defining ‘Future’ – A Period of Time Following the Moment,” and Prof Paquet concluded with “A Place We Call Home – Why AD Care Needs to Move Towards Home Care.”
The Need for Early Diagnosis
Doctor Marwan Sabbagh and Mister Ian Kremer
Lack of or delayed diagnosis represents a substantial unmet need in AD. In Europe, around one-half of all cases are estimated to be without a formal diagnosis, with figures of nearly two-thirds in North America and over 90% of people in Asia. Rates vary greatly though by region, country, setting, severity, and age.2 While some factors contributing to delayed diagnosis may be attributable to the individual or their caregiver not seeking an explanation for symptoms, large contributions are due to HCP and/or healthcare systems not providing or offering diagnostic services.3
According to Dr Sabbagh, early diagnosis of AD or mild cognitive impairment (MCI) could help ease the later burden on healthcare systems and optimise screening. It could also improve population health through better understanding of risk factors and through the use of biomarkers that could be applied clinically.
There is a misconception among some HCP, according to Mr Kremer, that until there is a cure or effective treatment for AD, there is no point in diagnosing it. However, people with AD and their caregivers report that they would have preferred that the AD diagnosis was given earlier than when they had received it.3
Mr Kremer noted that without a diagnosis, individuals are not able to face their possible future and plan accordingly, as well as allow friends and families to understand why their behaviour and health may be changing and offer their support. Withholding a diagnosis is also paternalistic, unethical, and can deepen health disparities. “If you convey the truth of AD in a timely, accurate, compassionate, and actionable way,” Mr Kremer stressed, “you empower patients, you destigmatise what they face, and you give them the tools to improve their QoL.”
Early diagnosis of MCI/AD is also essential as comorbid illnesses may both contribute to MCI/AD and be complicated by undiagnosed disease. For instance, Mr Kremer discussed that diabetes, a common comorbidity, and its associated sequelae can be better managed if the standard diabetes care plan is modulated to account for the presence of AD. For example, extra measures may be needed to remind a person with AD to control their diabetes, which can help prevent blindness or limb loss, the presence of which could hamper the QoL for a person with AD.
The Need for Timely Screening and Diagnosis
Doctor Marwan Sabbagh, Mister Ian Kremer, and Doctor Carla Abdelnour
“Overall,” said Dr Sabbagh, “we still don’t feel comfortable making a diagnosis of AD; therefore, we are delaying the diagnosis, access to treatments, and how we transform care.” Though there are many instruments for screening, diagnosing, and tracking MCI/AD, most are only performed periodically, some can be time consuming, and a few can be negated by rater variability.4 According to Dr Abdelnour, many screening tools are also misused and misunderstood.
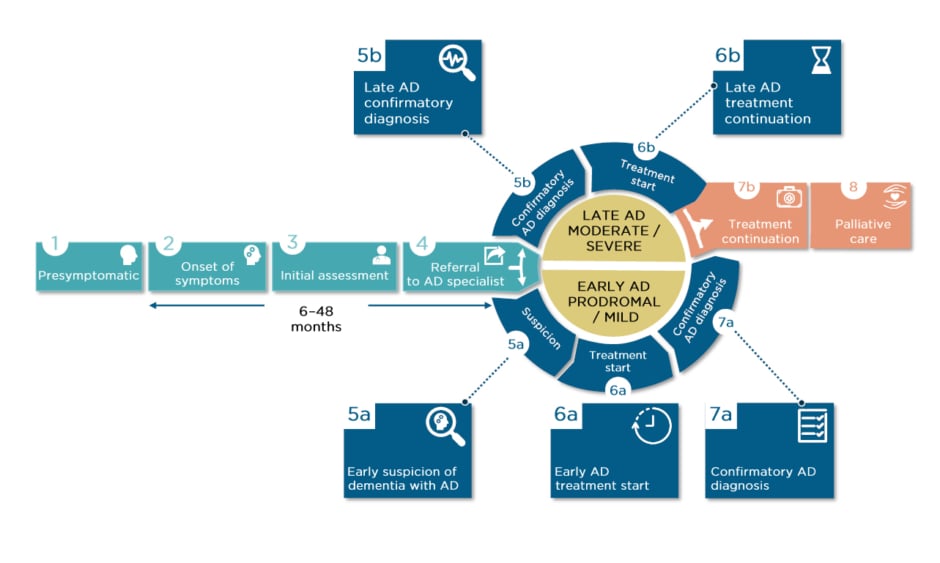
There may be years between presymptomatic initial development of AD-related neuropathology, MCI/AD symptom onset, and initial assessment, which wastes valuable diagnosis time (Figure 1). Dr Sabbagh reported that some early symptoms may even be dismissed by a primary care HCP, or misdiagnosed as depression, sleep problems, or normal ageing. There can also be long periods between initial assessment and referral to an AD specialist. With reference to Figure 1, Dr Sabbagh pointed out how it may not be until early suspicion of AD (Step 5a) when diagnostic tools are used. However, biomarkers, screening tools, and tracking software can be used much earlier, as soon as symptoms are noticed or even in presymptomatic stages.
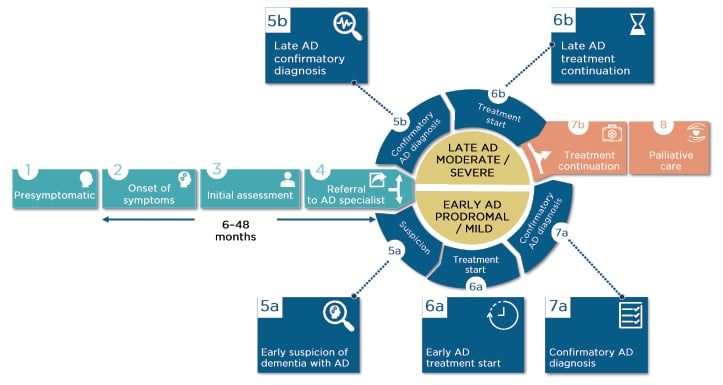
Figure 1: The patient journey.
AD: Alzheimer’s disease.
Adapted from the patient journey developed by F. Hoffmann–La Roche (data on file) in collaboration with Alzheimer’s Disease International (ADI)1 and Alzheimer Europe (AE).3
Currently, along with taking a detailed history, an HCP may use ‘bedside’ screening tools, carry out a physical and neurological exam, and look to blood tests to rule out other potential diagnoses.
Though biomarkers are increasingly used in clinical trials, biomarkers for diagnosis are still only advised, not mandated, and are not considered essential. However, as amyloid beta (Aβ), tau, and brain structure alterations can occur years prior to cognitive and clinical impairment, better understanding of the many evidence-backed, robust biomarkers for detection of presymptomatic changes is vital.5
Biochemical and Imaging Biomarkers
Dr Sabbagh discussed that what is needed for early diagnosis of MCI/AD is a combination of imaging of neurodegeneration, carried out using structural MRI and CT scanning, and direct markers of Aβ or tau via PET and cerebrospinal fluid (CSF) and/or blood plasma analysis.6
A key feature of AD is low levels of the Aβ isoform Aβ42 in the CSF, which is thought to be sequestered into amyloid plaques.6 This biomarker is highly sensitive for identification of amyloid pathology and, in combination with tau biomarkers, is predictive of who with MCI will progress to AD.7,8 Other amyloid CSF biomarkers include those identifying the Aβ40 isoform and those that assess the Aβ 42/40 ratio.9
Many companies have assays for CSF biomarkers, though not all are approved in all countries. These include the Elecsys® β-Amyloid (1–42) CSF assay (Roche Diagnostics, Rotkreuz, Switzerland), as well as ones for phosphorylated and total tau (Elecsys Phospho-Tau [181P] and Elecsys Total-Tau CSF assays, both Roche Diagnostics). Identification of phosphorylated tau levels is useful because it can predict progression from MCI to AD and differentiate between AD and frontotemporal dementia (92% specificity) and dementia with Lewy bodies (64% specificity).10,11 While total tau can also be used as a biomarker, it is not specific to AD as it will detect other forms of brain injury.6
There are further biomarkers in development, including those for the amyloid precursor protein and neurofilament light polypeptide, both found at different levels in those with AD, and plasma biomarkers, including Aβ isoforms, tau, and changes in phospholipids.9,12
Why Use Alzheimer’s Disease Biomarkers?
Dr Sabbagh highlighted how “measuring biomarkers with CSF immunoassays increases the certainty of a diagnosis of AD and can help evaluate progression.” There are, he pointed out, a number of myths around AD that hamper an HCP from using biomarkers; however, he posited, because CSF testing is commonly used for other conditions such as multiple sclerosis and meningitis, why shouldn’t they be used for MCI/AD?
The first myth about AD is that it is inevitable, with nothing to be done to reduce the risk. However, as will be discussed below, many cases of AD might be preventable through simple lifestyle adjustments.13-15 Secondly, some may think that biomarkers are not needed because a clinical diagnosis of AD alone is enough. However, current clinical diagnostic accuracy is only 77%, even among experts.16
Thirdly, there are myths around the current use of biomarkers, such as the belief that nobody else is using them and that test results take a long time to be received. However, Dr Sabbagh reported that in many parts of Europe, CSF testing is routine practice and that automated CSF assays such as Elecsys provide results on the day that they are analysed.
The final myth is that testing is too expensive. A recent UK study estimated that annually, if 100,000 extra amyloid PET scans were performed to enable a more precise and earlier diagnosis, it would cost £113 million. The same number of extra CSF tests, which can give information about both amyloid and tau, would cost only £48 million.17 Developments with the use of plasma biomarkers may further decrease costs.18 Ultimately, an early diagnosis, Dr Sabbagh emphasised, could not only have the potential to delay or prevent decline, give more access to new treatments, and improve QoL, it could also, in the long run, reduce total costs.
The use of biomarkers could also help change the diagnosis of AD from a diagnosis of exclusion to a diagnosis of inclusion. Dr Sabbagh suggested that in the future there would be a tiered approach to diagnosis, first with the use of plasma assays, then, if that is positive, CSF or PET testing to improve diagnostic accuracy.
Prevention of Mild Cognitive Impairment/Alzheimer’s Disease
Doctor Carla Abdelnour
The 2020 Lancet Commission report on dementia prevention, intervention, and care revealed that nearly 40% of all dementia cases might be either preventable or have their onset postponed.13 Protective measures include childhood education alongside lifelong physical, cognitive, and social activity. Potentially modifiable risk factors include comorbid conditions, such as hypertension, hearing loss, obesity, depression, diabetes, and dyslipidaemia, and lifestyle factors such as a poor diet, alcohol misuse, and smoking.13,19
Dr Abdelnour proposed that we could each have a ‘fingerprint’ of modifiable factors to use as a tailored prevention strategy. Early use of screening tools and biomarkers could not only identify those at potential risk of developing AD, but could potentially decrease that risk through interventional measures at an early stage. For instance, studies involving physical and cognitive training, along with a healthy diet and smoking cessation, have shown such interventions led to improvements in cognition and reductions in the risk of developing dementia and dementia incidence.14,15,20
Living with Mild Cognitive Impairment/Alzheimer’s Disease
Mister Ian Kremer and Professor Claire Paquet
Many healthcare systems are not prepared for the huge surge predicted in the number of AD cases globally. According to Mr Kremer, health systems need to be built, operated, and expanded to encompass the needs of people living with AD. “This includes helping legislators and decision makers understand what AD is and isn’t; what it does to the individual, the family, and to society; and how policy makers can change that future.”
Early diagnosis alleviates stress and potential family discord about previously unexplained symptoms. It also enables people living with MCI/AD to make legal, financial, caregiving, and other plans that will enrich their QoL in the near and long term. Additionally, if an accurate diagnosis is made and conveyed early enough in the symptomatic progression of disease, the individual living with AD will be capable of making their own decisions about end-of-life planning.
In a purely medical context, informing the person with AD and caregivers of the diagnosis enables them to make decisions about symptomatic treatments currently available for AD and comorbid conditions and evaluate potential participation in clinical trials. Each of these decisions, reported Mr Kremer, is momentous, a matter of individual dignity and autonomy, and made possible only if the HCP provides a timely, accurate, compassionate, and actionable diagnosis.
Home Assessment
According to Prof Paquet, three points have to be considered for the care of people with AD: 1) at-home clinical evaluation to get a real idea of a person’s degree of autonomy; 2) treatment options and re-adaptation of daily life; and 3) clinical and therapeutic follow-up.
While clinic-based assessments can reveal some issues related to AD symptoms, as currently around 60% of people with AD live at home,21 a home-based assessment is vital to best evaluate how a person goes about their daily life. According to Prof Paquet, this should occur as early as possible from symptom onset and continue through all stages of the journey as “the real objective of management and treatment is to preserve the autonomy of the patient in their home.” (Figure 1).
Compared to questionnaires and carer reports, a home evaluation is linked to a person’s real autonomy and disease severity and provides unbiased quantification of their difficulties and abilities. This is useful for not only the affected person, but also their caregivers and HCP, and can be used for both symptom assessment and intervention monitoring. Early detection and diagnosis could even help a person with MCI set up their home in a way to help delay AD.
As an example of what’s involved in a home-based evaluation, Prof Paquet discussed the ‘Equipe Spécialisée Alzheimer’ mobile teams in her home country, France. Over 15 sessions, the team not only carries out typical AD-associated neuropsychological testing, but also assesses multiple activities of daily living, such as how a person functions and copes in their kitchen and when outside in their neighbourhood. They also evaluate the carer’s help and awareness of the individual’s situation.
Such evaluations have led to several observations. For instance, the team have seen discordance between clinic and home-based evaluations, whereby a person deemed to have MCI no longer fulfils that diagnosis or where someone with moderate AD has acceptable autonomy in daily life using routine actions that were not shown in the clinic. There are also several aspects of daily life that cannot be explored in a consultation, such as a person’s ability to pay for shopping or access their home using a key, as well as carers’ awareness of a person’s ability levels at home. All of these factors may lead to changes in diagnosis, staging, management, and care needs.
Digital Medicine
Many different types of digital medicine initiatives are being explored that can help a person with AD remain in their home for as long as possible, as well as playing a role in diagnosis and monitoring. Digital medicine technologies include anything from minimal phone apps to social support robots.22 According to Dr Abdelnour, digital monitoring is not just for the HCP, but can be a crucial self-monitoring tool for an individual to know their own symptoms and track them over time.23
While digital medicine might seem like a future prospect, in diseases such as diabetes, use of a semi-implanted continuous glucose monitor can already provide information to someone and relay it back to the HCP’s office in real time via the use of an app.24 Such remote monitoring for people with AD could include providing data on cognitive, functional, behavioural, and physical factors. Current devices include sensors, such as a wrist-worn monitor, or implantable devices, as above. Their advantage is that not only is the information they gather accurate and reported over time, but that their use is relatively easy to implement and can be widely distributed.24-26
The range of useful digital technologies is large and adaptable to the person, need, and setting, including use of interactive voice response system phone calls, personal digital assistants, short message service texts on a mobile phone, smartphone apps, and computer programs. Feedback from an HCP on information gathered can be given either to the individual face-to-face or via programmed technology.23 Electronic devices are also useful in daily life to plan activities and remember crucial information such as appointments, meal times, bathing, and taking medication. Home-based technology is additionally being used to help people communicate with their families and avoid social isolation.22
Diagnosis and Symptom Tracking with Digital Medicine
Several platforms exist to evaluate the cognitive status of a person, such as CogniFit and Neuropsychology Online.27,28 These are not only useful for assessment, but can also be used to evaluate ongoing treatment efficacy and safety in both healthcare and clinical trial settings. As an example of a memory test app, Prof Paquet said that her work uses MemScreen, a 2-minute test that provides evaluation of memory and executive functions and can be carried out in any setting by the person affected or a caregiver. Tasks include object identification, calculations, timed reading, and a 12-word recall test. The app gives a memory score with normative results, an executive score, the total score, and an assessment of cognitive speed.
Another programme, the Face-Name Associative Memory Exam (FNAME), is a verbal episodic memory test, originally developed as a paper test for use in imaging studies.29 More recently, it was converted to a digital version and Dr Abdelnour reported how it has been found to be a sensitive tool for detection of MCI and highly correlated with the original paper test.30 Importantly, it has excellent acceptance by users, which is useful as people need to be engaged in the task as, if bored, results could be misleading.
Another app discussed was Floodlight (Genentech, a member of the Roche group, San Francisco, California, USA). While this has been developed for use by people with multiple sclerosis to track symptoms, a similar app could be useful for people with AD. The app contains a range of active and passive monitoring tests that measure how a person performs simple tasks using their smartphone, such as squeezing a tomato (to measure changes in hand-eye co-ordination and fine motor skills) and drawing a shape (to measure speed and accuracy of hand and finger movements), their level of mobility, including a 2-minute walk (to measure stamina and mobility), and their daily mood.31
Dr Abdelnour proposed that digital medicine devices can not only help with diagnosis, but can also aid an HCP and the person with MCI/AD in understanding disease progression “to enable them to accurately identify changes and engage in patient-led discussions.” She assured that such devices “will never replace an HCP; rather, it will bring together the patient and HCP in a more personalised relationship.”
Alzheimer’s Disease, COVID-19, and Remote Medicine
The coronavirus disease (COVID-19) pandemic has had a particularly brutal impact on people living with AD. From March to June 2020 in the UK, nearly one-half of those who died with COVID-19 in care homes had dementia including AD.32 With access to care homes restricted, people with dementia were reported as feeling ‘confused and abandoned’ because they were unable to see family or friends, with many care home managers reporting deteriorating health and wellbeing of their residents because of loss of contact with loved ones.33
For all people with AD, Mr Kremer emphasised how “standard precautions to prevent the spread of COVID-19, such as wearing a face mask, thorough hand-washing, and maintaining physical distancing, need to be adapted or better supported in light of an individual’s memory, decision-making, and judgement challenges.”
The COVID-19 pandemic has accelerated the use of and need for remote medicine. Prof Paquet highlighted how digital technology has been of particular use as an alternative to people visiting their hospital. For these people, her team have used online evaluation, such as the digital tools discussed above, to diagnose, measure symptom evolution, and assess the psychological impact of COVID-19 social distancing measures. Services in Dr Abdelnour’s home country, Venezuela, have rapidly implemented a coronavirus chatbot to help combat spread by providing users with a risk score based on location and symptoms.34
Conclusion
Mr Kremer’s final call to action was for HCP to work with colleagues in their own practice, locally, and globally, to help them to understand “the vital and central importance of early detection and diagnosis that is timely, accurate, compassionate, and actionable.”
People caring for those with MCI/AD, Mr Kremer stressed, “must work together and proactively preserve what makes people who they are.” He hoped that HCP would commit to “using all the available validated tools for detection and diagnosis and for advancing care and QoL for people living with AD.”