Meeting Summary
This symposium took place at the 4th Congress of the European Academy of Neurology (EAN) 2018 in Lisbon, Portugal, and focussed on the effective management of fluctuating Parkinson’s disease (PD). Prof Poewe introduced the topic by explaining how response fluctuations, including wearing-off, remain a key priority in the effective management of PD. Wearing-off fluctuations are often categorised as motor or non-motor, but the reality is that patients are frequently affected by both, with a significant impact on daily activities and quality of life. Prof Stocchi went on to explain that management strategies include adjunct therapies with catechol-O-methyl transferase (COMT) inhibitors, monoamine oxidase (MAO)-B inhibitors, and dopamine agonists. Clinical experience shows that within a few years most patients will be receiving a cocktail of these drugs to manage PD symptoms. Although many antiparkinsonian drug classes have overlapping indications, they have distinct mechanisms of action that can complement each other. Opicapone is a third generation, highly potent and effective COMT inhibitor that received European Union (EU) market approval in 2016 as an adjunct to levodopa for PD patients experiencing response fluctuations. While the efficacy and safety of once-daily opicapone have been proven in clinical studies, Prof Ebersbach gave an overview of real-life data from his own clinics, which show that the benefits of opicapone can be observed within 3 days of treatment initiation. The final presentation from Dr Morgante considered the management of non-motor symptoms in PD. Classically, these non-motor symptoms have been managed as non-urgent symptoms but are now recognised as a significant source of disability. It is vital for clinicians to recognise that many of these symptoms respond to treatment.
Introduction
Professor Werner Poewe
PD is a progressive neurodegenerative disorder with a long course of evolution. It is now understood that there is a long prodromal period when the typical motor system symptoms of the disease are not yet sufficiently progressed to make a clinical diagnosis.1 Most people with PD are treated as soon as they are diagnosed, often with levodopa or other monotherapies (such as MAO-B inhibitors or dopamine agonists). At this early stage, most patients enjoy stable, near-normal function with their first treatment. However, with continued disease progression, patients leave the so-called ‘honeymoon’ phase and enter a more complex treatment phase, during which they can experience a variety of response fluctuations (both motor and non-motor fluctuations) and dyskinesias.1 This is a crucial stage for disease management, and this symposium reviewed current best practices for symptom management during this complex stage of the disease.
Current Treatment of Fluctuations
Professor Fabrizio Stocchi
The terminology of ‘ON and OFF’ effects and motor fluctuations was originally coined by Prof David Marsden >40 years ago.2 While often considered as just one type of motor complication, the term ‘motor fluctuations’ actually covers a range of problems, including end of dose wearing-off, delays to ON, dose failures, and sudden OFFs.3 End of dose wearing-off is often the first type of motor fluctuation to emerge and can be said to herald the start of the patients’ transition to a more complex phase of the disease.4 When patients develop signs of wearing-off, rather than experiencing the consistent and sustained response to levodopa that they had previously exhibited, they often start to notice subtle fluctuations in a variety of signs and symptoms. While they still show a good response to their levodopa dose, they typically notice a re-emergence of symptoms before their next scheduled dose.
Even today, there is no universally accepted definition of wearing-off to use in clinical trials, and this has led to a range of incidence estimates, from 40% within 4–6 years of starting levodopa treatment5 to approximately 20–40% of patients within 2.5 years.6 Ultimately, >90% of PD patients will experience levodopa motor complications,5 and in young-onset PD the prevalence is even higher (>90% at 5 years).7 In recent years, it has become clear that the risk of developing wearing-off (and dyskinesia) is closely linked to levodopa dosing.8 Experts now advocate using the lowest dose of levodopa that provides satisfactory clinical control to prevent and delay the risk of both wearing-off and dyskinesia. Specifically, caution is advised when increasing the levodopa dose in large increments, and when considering doses >400 mg/day.8 Once motor fluctuations develop, the symptoms quickly become a priority in disease management because they are disabling. Patients say motor fluctuations are the most problematic part of their disease,9 and they have been shown to be a significant predictor for worse quality of life.10
Symptoms of Wearing-Off
It is important for clinicians to recognise that the first signs of wearing-off are neither well established nor the same for all patients. For example, while some patients may notice a return of their classic parkinsonian motor symptoms, (bradykinesia, tremor, and/or rigidity), others may first notice the return of non-motor symptoms, such as pain, anxiety, fatigue, mood changes, difficulty in thinking, restlessness, sweating, or sialorrhea.6,11 The subtleties of the symptoms mean that they may often go unrecognised by physicians and, therefore, remain untreated until they become prominent and disabling. Unless directly questioned, patients may not understand that the symptoms they are experiencing are because of their PD and instead attribute them to old age.12
Aetiology of Wearing-Off
In PD the loss of striatal dopaminergic neurons leads to a diminished ability to form, store, and regulate the release of dopamine. Accordingly, the brain becomes increasingly dependent on the availability of exogenous dopamine from levodopa.13,14 With progressive neurodegeneration, it is believed that fluctuations in plasma levels of levodopa (with its short elimination half-life of 1.0–1.5 hours) are increasingly translated into fluctuations in striatal dopamine receptor activation and deactivation.4,13,14
It is believed that this pulsatile stimulation of dopamine receptors in the striatum causes further destabilisation of the basal ganglia network, leading to changes in gene and protein expression and alterations in intracellular gene and signalling pattern. Ultimately, these molecular abnormalities are believed to be translated into altered patterns of neuronal firing in basal ganglia output neurons, resulting in the development of motor complications.4,13,14
Management Approaches
There are many different approaches to the management of wearing-off. Most traditional treatment strategies, while effective in the short term, do not address the aetiology of wearing-off and therefore require frequent modifications, causing an unnecessary burden for patients and their caregivers.15 Modification strategies of conventional levodopa formulations are the most commonly used approach because they are inexpensive. They include fractionation of the levodopa-dosing regimen, increasing the total dose of levodopa, changing the levodopa formulation (e.g., to controlled-release levodopa preparations), and the use of adjunctive therapy. While dose fractionation can reduce fluctuations in the short term, this approach does not eliminate troughs in levodopa plasma levels, which translate into a variable clinical effect15 and may reduce treatment adherence due to the inconvenience of additional daily doses.16 Continually increasing the size of individual doses of conventional levodopa usually leads to increased peak-dose dyskinesias and does not usually lead to any improvements in antiparkinsonian efficacy.15
Adjunctive therapy with MAO-B inhibitors (including selegiline, rasagiline, and safinamide) or dopamine agonists (e.g., pramipexole, ropinirole, and rotigotine) can also be used to reduce OFF time. These adjunct therapies may also allow for a reduction in levodopa dosage.17,18 However, because these drugs do not change the pharmacokinetic profile of levodopa, they cannot address the underlying cause of wearing-off.15 For this reason, COMT inhibitors (such as entacapone and opicapone) are often used as first-line therapy for wearing-off. COMT inhibitors act directly to reduce the metabolism of levodopa, thereby directly increasing the half-life of levodopa and decreasing the peak–trough variations in levodopa plasma levels that are associated with motor fluctuations.19 The COMT inhibitor tolcapone is typically only used as a last-line adjunct therapy due to the risks of liver toxicity, which necessitate regular monitoring of liver function.20
As the patient’s symptoms progress, most antiparkinsonian medications can be added to the patient’s current therapy regimen in order to maximise motor efficacy and keep overall tolerability at a safe level. Clinical experience shows that within a few years most patients will be receiving a cocktail of therapies to manage their symptoms. Although many antiparkinsonian drug classes have overlapping indications, they have distinct mechanisms of action that can complement each other. While they are very efficacious, caution is advised with the dopamine agonists due to their propensity to cause problems, such as oedema and impulse control disorders;21 patients should therefore be monitored frequently for any issues. When this adjunctive approach is no longer efficacious, advanced, surgical treatments (such as apomorphine infusion, levodopa infusion, or deep brain stimulation) should be considered.18,22
Managing Motor Fluctuations: A Case-Based Perspective
Professor Georg Ebersbach
Opicapone: A Once Daily Catechol- O-Methyl Transferase Inhibitor
Opicapone is a third generation, highly potent and effective COMT inhibitor that received EU market approval in 2016 as an adjunct to levodopa for PD patients experiencing response fluctuations. Based on research into the structure and function of the COMT enzyme, and using an analogue-based research approach, opicapone was specifically designed to reduce the risk of toxicity and improve peripheral tissue selectivity compared with other COMT inhibitors.23
In two pivotal studies, double-blind treatment with opicapone (25 and 50 mg) significantly reduced absolute daily OFF time by approximately 1 hour versus placebo.24,25 Reductions in OFF time were mirrored by significant increases in ON time without troublesome dyskinesia. In the BIPARK-1 study,24 the inclusion of entacapone as an active control helps understand the potential differences between opicapone and entacapone (with the caveat that the study was not set up for a head-to-head comparison). For example, responder rates for having at least 1 hour of reduced OFF time or at least 1 hour of increased ON time were significantly higher for the opicapone 50 mg dose (OFF time, p=0.0011; ON time, p=0.0028) compared with the placebo, but this was not the case for entacapone.24 Moreover, in the BIPARK-1 open-label extension study, patients who switched from double-blind entacapone treatment to open-label opicapone treatment experienced an extra reduction in OFF time of -39.3 minutes (p<0.05), with a corresponding increase in ON time without dyskinesia of 45.7 minutes (p=0.0148).26 For those patients who received double-blind opicapone 50 mg treatment, the magnitude of efficacy benefit was maintained throughout the 1 year open-label treatment phase.26
Real-life experience with opicapone has also been positive. A recent prospective observation of 88 in-patients with PD (mean age: 68.8 years, 73.5% male) treated at a single expert site between October 2016 and June 2017 found early benefits of opicapone within 3 days of treatment initiation. The study included 52 patients previously treated with entacapone who required a change due to lack of efficacy or intolerability (e.g., diarrhoea) and 8 patients previously treated with tolcapone.27
Overall, the majority of patients reported improvement of clinical status (on clinical global impression of change) with opicapone initiation, with around 1 in 10 patients reporting no change after 3 days and the same proportion reporting worsening (Figure 1A). When analysed by previous COMT therapy, 44.2% of patients previously treated with entacapone and 37.5% of patients previously treated with tolcapone showed an improvement in clinical global impression of change scores (Figure 1B). However, 16 patients (18.2%) discontinued opicapone therapy: 4 due to side effects, 5 due to lack of efficacy, and 7 due to miscellaneous reasons. Overall, 6 of the 88 (7%) patients had hyperkinesias within the first 3 days of opicapone initiation, which were generally manageable through levodopa dose reductions. Thirteen patients reported hallucinations, which is more common than in the pivotal trials but reflective of the neuropsychiatric and other comorbidities in this real-world sample. These were not a common cause for discontinuation, and those patients who were known to be prone to hallucinations could be managed by reducing the levodopa dose before starting opicapone therapy. Levodopa dose reductions were easily conducted because patients only took one daily dose of opicapone, and clinicians could simply reduce the dose of levodopa according to individual patient needs without worrying about how to titrate the dose of COMT inhibitor.27
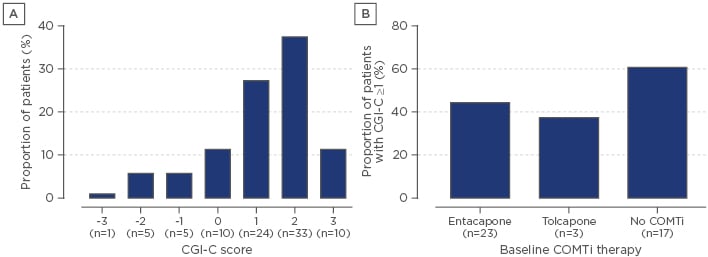
Figure 1: Clinical global impression of change at 3 days post initiation of opicapone adjunct therapy (A) overall and (B) according to previous catechol-O-methyl transferase therapy.
CGI-C: clinical global impression of change; COMTi: catechol-O-methyl transferase inhibitor.
Adapted from Gandor et al.27
Facing the Challenges of Non-Motor Symptoms
Doctor Francesca Morgante
It is now accepted that non-motor symptoms are part of the PD ‘syndrome’.28,29 It is also now better understood that PD follows a clinical pattern, with a range of non-motor symptoms defining the premotor phase.30 The development of many of these non-motor symptoms are consistent with the Braak pathology staging system, in which Lewy bodies appear to start from the dorsal motor nucleus of the vagus nerve, the olfactory bulb, and the enteric nervous system, later spreading to the substantia nigra, areas of the midbrain, and basal forebrain.31 Indeed, it is now suggested that the vagus nerve and the gut–brain axis may act as the generator of the pathological process in PD.32,33 Research is ongoing to evaluate whether clinical subtyping according to the types of non-motor symptoms experienced can help predict the longitudinal prognosis for people living with PD.28,34,35 For example, researchers are now trying to identify those patients most at risk of developing impulse control disorders,36 hallucinations,37 and cognitive impairments or dementia.38 Such information will help clinicians with decision-making, as the presence of these neuropsychiatric symptoms often modifies treatment decisions (e.g., avoid dopamine agonists or advise cognitive training strategies).
Classically, non-motor symptoms have been managed as non-urgent symptoms, but when present they are a significant source of disability and are associated with a worse quality of life.39-41 It is vital to recognise that many of these symptoms respond to treatment. They may be related to the patient’s OFF state (i.e., they are a non-motor fluctuation), in which case they may respond to optimisation of dopamine therapy, or they may be dopamine-independent and require separate management.
Conclusions
Despite all the many recent advances in the medical management of PD, the management of motor fluctuations remains a key issue for many patients. Wearing-off can emerge early in the patient journey, and clinicians should be proactive in asking the patient about any symptoms that begin to emerge when their levodopa dose is losing effect but disappear when they take their next dose. When weighing the risks and benefits of potential medications, treating clinicians should consider the full spectrum of motor and non-motor symptoms that the patient experiences. Combinations of therapies may make best use of the various modes of action available, but practical considerations, such as pill burden and ease of titration, should also be considered.








