Meeting Summary
This symposium was held at the Muscular Dystrophy Association (MDA) Clinical & Scientific Conference 2025, in Dallas, Texas, USA. Speakers gave an overview of the patient experience and natural history of Becker muscular dystrophy (Becker) and presented the latest Phase I/II clinical data for the novel investigational agent, sevasemten.
Becker is a serious, rare, neuromuscular disorder that produces irreversible muscle loss and progressive impairments in mobility and cardiac function, amongst other physical and emotional effects. However, there are currently no approved treatments for Becker.
In a powerful start to the symposium, Michael Voto, New Jersey, USA, a patient advocate living with Becker, provided insight into the lived experience of Becker and, together with Abby Bronson, Vice-President of Patient Advocacy, Edgewise Therapeutics, Colorado, USA, highlighted the importance of the patient voice to improve awareness, and create consistent standard-of-care in Becker. Craig McDonald, Director of the MDA Neuromuscular Diseases Clinics, University of California Davis, USA, explained how understanding of the natural history of Becker can better direct the use of functional measures such as the North Star Ambulatory Assessment (NSAA), as a meaningful outcome in Becker clinical studies. McDonald also outlined clinical advancements with the fast myofibre (Type II) myosin inhibitor, sevasemten (formerly EDG-5506), including new data from CANYON, the largest randomised, controlled clinical trial in Becker to date. CANYON met its primary endpoint demonstrating significant decreases in biomarkers of muscle damage with sevasemten treatment, as well as stabilised ambulatory function (NSAA) trending to improvement, which is a clinically meaningful outcome for patients.
Introduction
Becker is a serious, progressive muscular dystrophy that presents predominantly in males due to an X-linked, recessive inheritance.1-3 Becker results from mutations in the gene encoding the protein dystrophin, which plays a role in the protection of skeletal and cardiac muscle fibres.4 The mutations are typically in-frame, leading to the production of a partially functional protein; without fully functional dystrophin, contraction-induced muscle injury accumulates and progresses, producing debilitating muscle weakness that profoundly impacts upon patients’ daily function and quality of life.3,4 Once disease progression starts, patients with Becker are on an irreversible path to loss of mobility, function, and independence.5,6 At present, no treatment is approved to amend or slow the course of Becker. Thus, the ongoing clinical trial programme investigating a novel agent, sevasemten, for the treatment of Becker, is of prime interest.
The Lived Experience of Becker Muscular Dystrophy
The Patient Perspective
“Patients are the experts on their disease, so it is important to include the patient voice in these discussions,” said Bronson, introducing patient advocate Voto, who spoke about the experience of living with Becker.
Although largely healthy in childhood, a Kindergarten teacher noticed that Voto was not able to keep up with other children. This led to two consecutive misdiagnoses: the first being Duchenne muscular dystrophy, and the second, at age 7 years, being simply told there was ‘nothing wrong’. Voto was eventually diagnosed with Becker 25 years later, in 1992, after experiencing knee pain: “[The neurologist] told me all this negative stuff and gave me a ton of prescriptions. So, I left his office, put the prescriptions in the garbage, went back to work and continued living my life.” However, in 2000, Voto noticed his health declining and experienced a major fall down 18 stairs. This time, a neurologist with a speciality in muscular dystrophy delivered a more constructive prognosis around Becker. “He was such a positive man,” recounted Voto. “He told me that I was probably going to have a full life, and likely wouldn’t stop walking. He was quite right. Though unfortunately, I suffered a fall when I was 65, and ended up in a wheelchair.”
Voto spoke about the impact of the symptoms of Becker on daily life following this decline in health: “It was harder to do my job, harder to go upstairs, and I became slower. I continued to work until 2008, when I started to have tremendous difficulty doing my job (as a field service technician), and my employers were really getting on my back. I left my career, and fortunately I was granted social security disability. I took a part-time job to keep busy, but 10 years on, I couldn’t continue as I couldn’t lift anything. Stairs became impossible. I could still maintain myself, but I was using a walking stick more and more. Then, my health really started to decline, and I had two major falls. Now, even having a shower has to be a whole planned activity.”
After diagnosis, Voto was self-motivated in seeking care and support: “I started researching my disease and became more aware of available resources. I started seeking physical therapy, which has been such an important part of my journey to maintain [my function]. I am very diligent about my cardiac care now, my physical therapy, and my neurology, and I find that a tremendous help. Thankfully, along with Medicare (health insurance), I’m able to afford the care I need, but it’s very difficult and expensive.”
Voto also revealed some of the personal burdens that have come with the increasing disability of Becker, such as difficulties seeing family regularly or socialising, a lack of independence, and a reliance on other people. Adding to this, the use of a wheelchair has led to exclusion due to transport and accessibility issues. Voto commented: “It’s not easy living with this disease. I’m always smiling, but there are times when it’s hurtful, disappointing, debilitating. You hide a lot of emotions. To have your freedom taken away is hard. It’s not a mild case of Duchenne, it’s different. We need a lot more information and awareness.”
Listening to Patient Needs
Voto’s testimony highlighted the importance of listening to the needs of the Becker community in order to direct education, support, and patient care. Bronson presented anecdotal evidence of how patients are often not motivated to seek care due to incomplete understanding or acceptance of their condition and may be unaware of the full array of potential Becker symptoms, such as cardiac involvement. Bronson also discussed data from a recent survey conducted in the USA among 50 adolescents and adults with Becker,7 which revealed unmet needs, including a long and frustrating road to diagnosis, and many unseen challenges. As Bronson elaborated: “We found that a third of patients were originally misdiagnosed and living with symptoms for years before receiving a diagnosis. One of the reasons for this is the lack of awareness of Becker in the community. Sometimes Becker is called the ‘invisible disease’. Interestingly, we found that Becker patients experience a lot of pain and fatigue, which can really impact their day-to-day life, but is not so visible to others. There is also the emotional strain of getting access to disability support, or navigating the educational or employment systems, which makes a big difference to a person’s quality of life.” Respondents in the survey also highlighted a desire for more opportunities to connect with others within the Becker community.
Another main theme that emerged was the variability in care provision and notable gaps, particularly as speciality care for Becker becomes less consistent in adulthood, a time when symptoms often escalate. Cardiac care and pain management were also identified as pressing health issues that require greater prioritisation and monitoring by healthcare providers. Almost half of people surveyed (44%) considered greater knowledge of Becker (disease traits, treatments, clinical trials, research, and education) as a fundamental need.7
Overall, listening to the voices of patients could help to improve education and activities around Becker, address gaps in care provision, and direct impactful, patient-centric clinical programmes.
Natural History of Becker Muscular Dystrophy
Recently, there have been great advancements in the clinical trial landscape and study of Becker natural history. As McDonald observed: “I’ve been looking after Becker patients for 35 years, and it’s remarkable the progress that has been made in just the last few years.” Elucidating the natural history of Becker is important for understanding disease progression and prognosis, managing patient care and discovering therapeutic targets. It also provides insights to help design and power clinical trials, and identify clinically meaningful measures of functional change.
The NSAA is a clinically meaningful measure of global motor function that has been used in Becker natural history studies,5,8,9 as well as in clinical trials, to longitudinally track functional status. It is a validated, composite measure that assesses motor function across 17 test items (higher scores equate to higher functional levels).5 McDonald explained how the NSAA items relate to daily life for a patient with Becker, reflecting their ability to take part in recreational activities (items: jump/hop/run); put on shoes and socks (item: stand on one leg); or get out of bed, use the toilet, or use public transport independently (item: rise from a chair). For someone with Becker, a one-point change in NSAA can mean the difference between independence and requiring assistance from another person or mobility aid. Natural history studies in Becker demonstrate an average decline of 1.0–1.7 points in NSAA annually.5,6,8,9
McDonald concluded that Becker is a serious muscular dystrophy and, once function begins to decline, individuals continue on an irreversible path to losing muscle, and consequently function. Stabilising function, or even reducing the slope of decline, is therefore an important and urgent goal in Becker.
Clinical Advancements with a Novel Agent: Overview of the Sevasemten Clinical Programme
Human skeletal muscles consist of ‘slow’ (Type I) and ‘fast’ (Type II) muscle fibres. Fast muscle fibres are more susceptible to the contraction-induced injury seen in Becker.10,11 “When dystrophic muscle undergoes significant stress and damage, the repeated contraction-induced injury impacts the muscle’s ability to repair itself.4,12,13 Eventually, the regenerative ability ceases, and the fibre dies and is replaced by fat and connective tissue,”6,12 explained McDonald. Sevasemten is a first-in-class fast skeletal (Type II) myosin inhibitor that is designed to protect against excessive contraction-induced muscle injury while preserving muscle function.14 The clinical development of sevasemten has advanced rapidly in recent years, and McDonald gave an overview of findings from the clinical programme thus far, including newly released data from the most recently completed CANYON study.
ARCH
The Phase I, open-label ARCH study assessed the safety and pharmacokinetics of sevasemten (10–20 mg daily) in 12 adult males with Becker and moderately severe functional impairment (median NSAA: 15.5).15,16 Although natural history predicted that these patients would show consistent decline, there were early, rapid reductions in biomarkers of muscle damage (creatine kinase [CK] and fast skeletal muscle troponin I, Type 2 [TNNI2]) sustained across 24 months of treatment.15 Notably, patients also showed stabilisation of function, with a mean change of -0.2 points in NSAA score at 24 months,15 diverging from the expected decline of -2.4 points.5,6,9 McDonald explained that prediction models for natural history trajectories can provide important benchmarks for the outcomes of patients receiving novel therapies. Patients in the ARCH study treated with sevasemten have been compared to this modelled, predicted decline in NSAA, which revealed statistically significant differences for ARCH patients versus the control group at 12, 18, and 24 months.17,18
DUNE
Exercise causes transient elevation in circulating muscle injury proteins, TNNI2 and CK, in people with Becker.19 The Phase II, DUNE exercise-challenge study, included nine adult patients with Becker who were randomised 2:1 to receive either oral sevasemten 15 mg daily or placebo for 16 weeks.20 During a period of normal activity, sevasemten-treated patients had a significant reduction in injury biomarkers compared to placebo (primary endpoint): CK decreased by 45%, and TNNI2 by 89%, versus baseline (p<0.01 and p<0.05 versus placebo, respectively).20 McDonald described these as “impressive biomarker results without exercise, after a relatively short duration of treatment.” At baseline and Week 12, patients underwent a vigorous exercise challenge of sustained cycling at 70% VO2 max (maximum oxygen uptake).20 In the 24 hours post-exercise, sevasemten significantly reduced the signature increases in CK seen at baseline (i.e. pre-treatment) by 49% (p<0.001) and TNNI2 by 75% (p<0.05).20 Sevasemten was also generally well tolerated over 16 weeks.20
CANYON
CANYON is the largest randomised, controlled study conducted in Becker to date.21 This multicentre, double-blind, placebo-controlled, Phase II trial evaluated the safety and effect of sevasemten on biomarkers and ambulatory function (NSAA) in patients with Becker over 12 months.21 The study enrolled ambulatory males aged 12–50 years with a dystrophin mutation and a Becker phenotype, and (for adults only) an NSAA of 5–32 points.21 Overall, 40 adults and 29 adolescents were enrolled, with results available for the adult patient group. Adults were randomised 3:1 to receive oral sevasemten 10 mg daily (n=28) or placebo (n=12). The primary efficacy endpoint was change from baseline in CK averaged across Months 6, 9, and 12; the key secondary endpoint was change from baseline to Month 12 in NSAA score.
Natural history data5 have shown that some Becker genotypes are ‘fast progressors’, generally corresponding to NSAA scores of 32 and below. Using this NSAA criterion as the threshold for entry into CANYON enriched the study population for those patients likely to progress over a 1-year period. Levels of functional severity were not well matched between groups at baseline, meaning that patients receiving sevasemten had greater functional impairment than the placebo group (mean NSAA, 18.4 versus 24.2 points, respectively).
The study met its primary endpoint (change from baseline in CK across Months 6–12), which McDonald described as “encouraging with regard to target engagement.” Sevasemten treatment produced a decrease from baseline in CK levels over 6–12 months, and was statistically significant versus placebo (Least squares [LS] mean between-group difference, -28%; p=0.02; Figure 1). Plasma TNNI2 also demonstrated a decrease, with an LS mean between-group difference of -77% versus placebo (p<0.001). In a wider examination of 26 muscle injury proteins found to be elevated/abnormal in patients with Becker, sevasemten broadly reduced circulating levels of these biomarkers after 1 month of treatment (and maintained through 6–12 months), while placebo had minimal effects on this panel of proteins.22
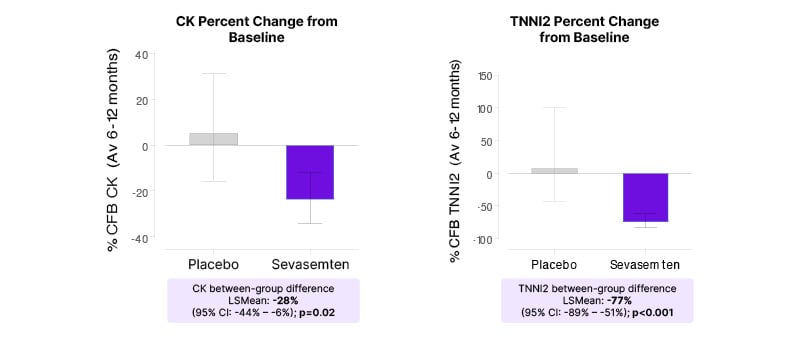
Figure 1: Rapid and sustained decreases in creatine kinase and fast skeletal muscle troponin I, biomarkers of muscle damage, in patients treated with sevasemten (primary endpoint; CANYON study).
CK and TNNI2 values were log-transformed. LS means, LS mean differences, and CIs were back-transformed
to percent scale.
Av: average; CK: creatine kinase; CFB: change from baseline; LS: least squares; TNNI2: fast skeletal muscle troponin I.
In the key secondary endpoint, function (NSAA) remained stable over the 12-month study period in patients treated with sevasemten, but declined in the placebo group in line with the predicted natural history of Becker (Figure 2). Although not powered to show a statistically significant difference versus placebo in NSAA, there was a positive trend in favour of sevasemten after 12 months of treatment: LS mean difference 1.12 points versus placebo (p=0.16). Moreover, in an NSAA responder analysis, 63% of patients treated with sevasemten showed stability or improvement after 12 months (odds ratio: 3.4; p=0.16), compared with only 33% of patients in the placebo group. Timed functional tests (4-stair climb; 100 m timed test; 10 m walk/run) also showed trends in favour of sevasemten at 12 months.
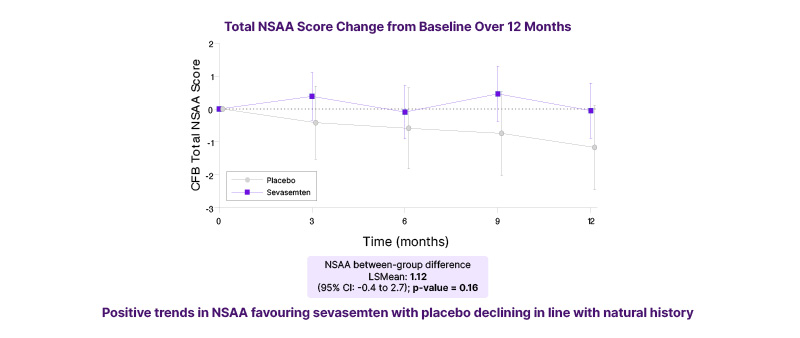
Figure 2: North Star Ambulatory Assessment function remained stable over time in patients treated with sevasemten for 12 monthsª (key secondary endpoint; CANYON study).
ªStudy was not powered to show a statistically significant difference versus placebo.
CFB: change from baseline; LS: least squares; NSAA: North Star Ambulatory Assessment.
Sevasemten was well tolerated in adults across 12 months of treatment in CANYON, with no safety concerns identified. The overall rate of treatment-emergent adverse events (TEAE; 92.9%, n=26/28) was similar to placebo (83.3%, n=10/12), and all TEAEs were mild or moderate in severity. The most frequent TEAEs (sevasemten versus placebo) were headache (32% versus 17%) and dizziness (32% versus 0%). McDonald commented, “Sevasemten has an excellent safety profile. In terms of individual TEAEs, there is a tendency towards headache, dizziness, and somnolence, which generally show improvement over time, but there appears to be no renal, hepatic, or cardiac toxicity with this compound.” In response to an audience question over cardiac effects, Donovan added: “We have looked at echocardiograms every 6 months as a safety measure, and cardiac function was stable over 12 months. The myosin in the heart is different from that in fast skeletal muscle fibres, so it is not anticipated that sevasemten would affect the heart.” Another question raised was whether exercise capacity or strength was compromised by sevasemten treatment. Donavan responded, “We do not see any changes in grip strength over the treatment periods in CANYON or in ARCH.15 Also, in DUNE we were able to measure maximum oxygen uptake both pre- and post-drug in the challenges, and there was no difference,20 so a protective effect was elicited without a negative effect on function.”
In summary, CANYON is the largest interventional trial in Becker to date, and the first to achieve its primary endpoint, with sevasemten achieving significant decreases in biomarkers of muscle damage and meaningful stabilisation of function (NSAA) in this progressive condition. Evaluation of the full adult and adolescent dataset from CANYON is ongoing.
Next Steps: Continued Investigation in Becker
Following the encouraging findings from the ARCH, DUNE, and CANYON studies, sevasemten continues to be investigated in Becker. GRAND CANYON is a Phase II, global, multicentre, placebo-controlled, pivotal cohort study, that is fully enrolled with 175 patients (NSAA 5–32).21 As supported by data from CANYON, the pivotal cohort’s primary endpoint is powered at >95% to demonstrate a statistically significant difference in NSAA between sevasemten and placebo groups at 18 months. Results are expected in late 2026.
MESA is a Phase II, open-label extension study to enable assessment of the long-term (3-year) safety and durability of sevasemten effects in Becker.23 The study is enrolling patients from the ARCH, DUNE, CANYON, and GRAND CANYON trials and, to date, 99% of eligible patients have enrolled in MESA. McDonald observed, “This speaks highly of the degree to which this medication is tolerated, and the positive effects these patients are feeling on the drug with respect to their disease stabilisation. I think MESA is really going to contextualise these sevasemten data versus the model data of long-term natural history.”







