BACKGROUND AND AIMS
Neuroendocrine neoplasms (NEN) represent a rare heterogeneous group with an increasing incidence, consisting of neuroendocrine tumours (NET) and neuroendocrine carcinomas (NEC), mostly prevalent in the gastrointestinal tract. Cancer stage, tumour grade, mode of treatment, and mainly early diagnosis affect overall survival rate. Their classification is based on their differentiation, grade, mitotic rate, and Ki-67 index status, and according to the latest 2019 World Health Organization (WHO) definitions, NETs are well differentiated, whereas NECs are poorly differentiated.1 Genetic analysis of NENs deriving mostly from pancreatic cases recognises differences in the mutational profiles between NETs and NECs, but there is still limited understanding on the molecular pathogenesis of the disease.2 The molecular mechanism of autophagy is crucial for the survival of cells. However, it holds a dual role in the progression of cancer, as it can either promote tumour survival by ameliorating stress, recycling unwanted proteins, and generation of energy; or suppress tumour survival through apoptosis, necrosis, and inflammation.3 Various molecules participating in the initiation, nucleation, elongation, or fusion of autophagosome with lysosome, are targeted to study autophagy. The LC3B protein, part of the elongation of the dynamic membrane structure of the autophagosome, is the most characteristic and widely-used autophagy marker, especially in association with the adaptor protein p62/sequestrome 1. Their interaction facilitates autophagic degradation of ubiquitinated protein aggregates in lysosomes.4,5
MATERIALS AND METHODS
In this study, autophagy flux was assessed by means of p62 and LC3B protein expression in various GEP-NENs derived from pancreas (n=19), stomach (n=2), small intestine (n=2), and oesophagus (n=1), where existing data are limited. The study was approved by the ethics committee of the Hippokration General Hospital of Athens, Greece. The patient cohort consisted of nine patients with NECs (six male, three females; mean age: 55±7; six pancreas, one stomach, one small intestine, one oesophagus), and 16 patients with NETs (11 male, five female; mean age: 58.69±3.7; 13 pancreas, one stomach, two small intestine). Immunohistochemistry was performed on 5 μm sections of formalin-fixed paraffin-embedded tissue from lesions and normal adjacent tissue. Staining intensity (negative to high: 0–3) was multiplied with the immunoreactive score (0–10%=1; 11–50%=2; 51–80%=3; 81–100%=4) to obtain the final score. Epithelial and stromal cell populations were assessed separately.
RESULTS
Results were statistically analysed using IBM SPSS Statistics 28.0 (Armonk, New York, USA). Both cytoplasmic and nuclear p62 expression were observed. Expression, mainly cytoplasmic, was higher in NEN lesions compared to their corresponding NA epithelium (0.92±0.4 versus 0.0; p=0.017), in which nuclear expression was prominent (p=0.047). LC3B expression was also induced in lesions compared to NA epithelium, but this difference was not statistically significant (p=0.1), probably due to the limited number of patients. Nevertheless, LC3B protein expression was mainly detected in NENs lesions’ epithelium with limited expression in stroma (5.67±0.9 versus 0.47; p<0.001). The difference between epithelium and stroma was more prevalent in NEC lesions (NECs: 7.25±1.5 versus 0.62±0.3; p=0.018) versus NETs (4.67±1.3 versus 0.58±0.3; p=0.016 [hl]Figure 1[/hl]).
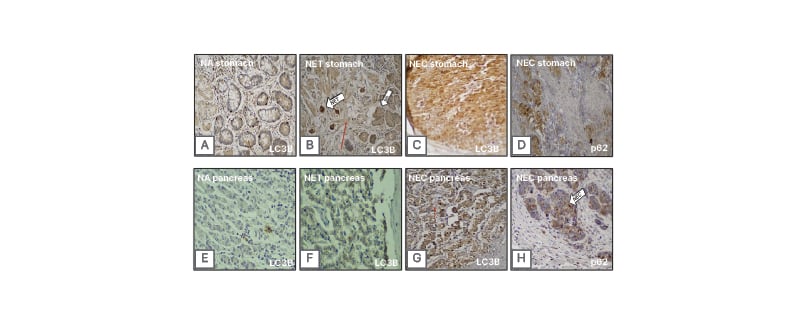
Figure 1: Representative immunohistochemical detection of LC3B and p62.
LC3B and p62 in stomach samples (A–D) and pancreas samples (E–H): p62 cytoplasmic expression is prominent in NEC lesions (D and H), while LC3B protein expression is significantly increased (A–C and E–G) in lesions’ epithelium compared to their corresponding stroma (epithelium versus stroma [red arrow]; p<0.001). This pattern is more prominent in NEC lesions (NECs: p=0.018 versus NETs: p=0.016). Magnification: 20x.
NA: normal adjacent epithelium; NET: neuroendocrine tumour; NEC: neuroendocrine carcinoma.
CONCLUSION
An apparent induction in autophagy was also detected in this study, which includes NENs from various organs of the gastrointestinal tract, and further supports existing data derived from pancreatic NENs.6,7 However, more data are required, as the number of patients in this cohort was limited. Still, the observed prevalence of autophagy in epithelium compared to stroma in NECs versus NETs might be related to their distinct clinical phenotype, making autophagy a potential candidate for introducing new therapeutic strategies for patients with GEP-NENs.








