At the 2018 European Society for Medical Oncology (ESMO) Congress in Munich, Germany, the Melanoma World Society (MWS) and European Association of Dermato-Oncology (EADO) presented the results of a survey assessing worldwide access to first-line recommended treatments for metastatic melanoma and the major determinants of access.
Metastatic melanoma is a chemotherapy-resistant cancer with a median survival of 6–9 months prior to 2010.1 In recent years, a major breakthrough was achieved with targeted therapy and immunotherapy, leading, for the first time, to significantly prolonged survival for this group of patients, with nearly 50% of patients in good prognostic groups surviving up to 5 years based on recent trials.2-5 However, despite the high efficacy of targeted therapy and immunotherapy, they have high costs, which has led to restricted access to these treatments in parts of Europe; in 2016, >5,000 patients did not have access to these treatments.6 Significant delays in reimbursement and different insurance coverage are some of the challenges healthcare systems face when trying to adapt to the rising costs of cancer care.6 In this setting, there is a clear need for an objective measurement of clinical benefit of every treatment and development of value-based pricing. The American Society of Clinical Oncology (ASCO) framework net clinical benefit 16 score and the ESMO magnitude of clinical benefit score have both been developed with the intention of being used for developing pricing and prioritisation of medicines for reimbursement and/or insurance coverage.7,8
The degree of inequality and major determinants of access to innovative treatments for metastatic melanoma have been largely unexplored. The MWS and EADO conducted a web-based survey on access to first-line recommended treatments for metastatic melanoma by current guidelines (National Comprehensive Cancer Network [NCCN], ESMO, and European Organisation for Research and Treatment of Cancer [EORTC]/EADO/European Dermatology Forum [EDF]) among melanoma experts from 1st September 2017–1st December 2018 from 34 countries: the USA, China, Australia, Argentina, Brazil, Chile, Mexico, and 27 European countries. Data on licensing and reimbursement of medicines and the number of patients treated were correlated with the data on health expenditure per capita, Mackenbach score of health policy performance, health technology assessment, and the ASCO and ESMO magnitude of clinical benefit scores of clinical benefit and market price of medicines.7-10 Regression analysis for evaluation of correlation between the parameters was carried out using SPSS software.
In this study, the estimated number of patients without access in surveyed countries worldwide was 10,131 (Table 1). The recommended BRAFi+ MEKi combination and anti-PD1 immunotherapy were registered and fully reimbursed in 17 (50.0%) of the countries, and anti-CTLA4+ anti-PD1 combination in 9 (26.4%) countries. In 14 (41.1%) countries, the majority of patients were treated according to the recommended guidelines. Median delay in reimbursement was 871 days (range: 0–1,274 days). These results were in correlation with ASCO (rho=0.819; p=0.004), and ESMO scores of clinical benefit (rho=0.933, p<0.01) and median market price (rho=0.694, p=0.026), as well as with health expenditure per capita, health policy performance scores, and health technology assessment implementation (p<0.05). The medicines with the highest scores of clinical benefits were the ones with the longest delay in access. In the majority of countries (64.2%) price negotiations or managed entry agreements with national authorities were necessary for reimbursement.10
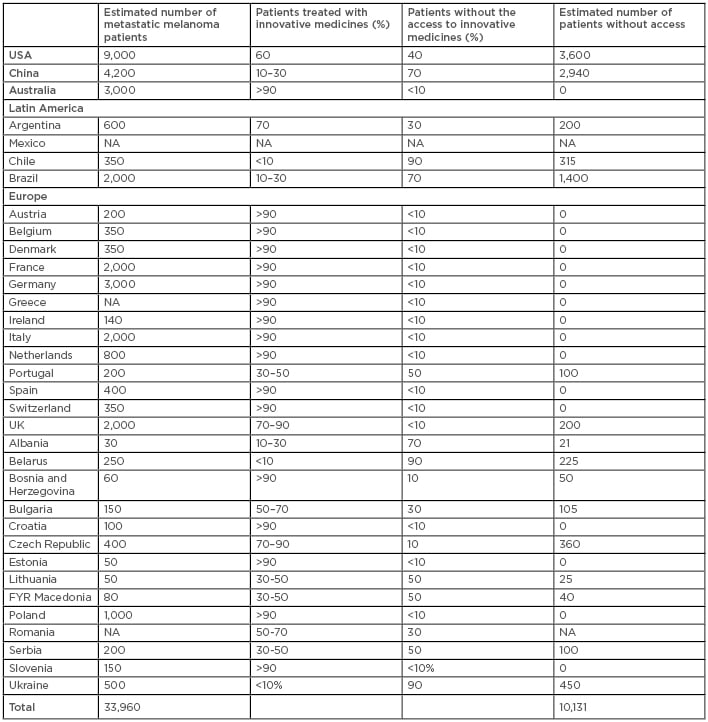
Table 1: Estimated number of patients without access to innovative medicines in surveyed countries.
NA: data not available.
In conclusion, great discrepancy exists in metastatic melanoma treatment globally. Access to innovative medicines correlated with economic parameters as well as with healthcare system performance parameters. Patient-orientated drug development, market access, and reimbursement pathways must be urgently found.








