BACKGROUND
Breast cancer is the leading cause of death in females aged 35–54 years and 15% of breast cancers first present in females of reproductive age.1,2 A trend of delaying childbearing to later ages, with falling birth rates in the <30 year olds and the average maternal age being >30 years,3 is likely to cause increasing rates of pregnancy-associated breast cancers (PABC).4,5 A national collaborative approach was used to evaluate the management of PABC in the UK; herein, the authors report the largest UK patient series of PABC.
METHODS
PABC cases (January 2010 to January 2020) were identified and demographic, tumour characteristic, oncology treatment, and obstetric data were collected retrospectively. Hospitals were recruited via collaborative research and trainee networks. Descriptive statistics were used to describe the treatment received, which were then compared between sites in and outside of London, UK.
RESULTS
Data for 57 patients from eight National Health Service (NHS) Trusts were included. The median age at diagnosis was 34 years, ranging from 24 to 43 years. Gestation of pregnancy at diagnosis ranged from 2 to 38 weeks. The majority of patients were diagnosed with early, localised breast cancer (97%), and 3% had metastatic disease. 58% of patients had oestrogen receptor positive (ER+) breast cancer, 34% were human epidermal growth factor receptor 2 (HER2) positive, and 32% were triple negative. Tumours were of histology Grade 2 in 25% and Grade 3 in 68%.
Surgery was performed in 95% of cases, with 40% receiving breast conserving surgery. All 57 patients received chemotherapy; the intention of treatment, pregnancy gestation, and choice of therapy is shown in Figure 1. Granulocyte colony stimulating factor (GCSF) support was prescribed in 39% to prevent neutropenia. Toxicity was reported in 70% of regimens, though only 1% reported a Grade 3 toxicity.
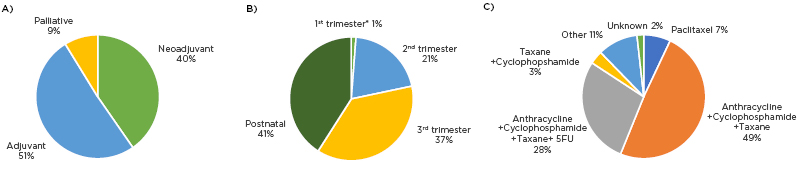
Figure 1: Chemotherapy treatment of PABC of A) intention of treatment; B) gestation of pregnancy at the start of treatment; and C) chemotherapy regimens prescribed.
*Patient scheduled for termination of pregnancy.
All ER+ patients received oestrogen receptor targeting therapy. All patients with HER2+ breast cancer received targeted therapy with trastuzumab (58%) or trastuzumab plus pertuzumab (42%) postpartum. No patients received radiotherapy whilst pregnant and 38 (67%) received it postpartum. Radiotherapy was delivered to the whole breast (27%), partial breast (2%), chest wall (34%), supraclavicular fossa (25%), axilla (3%), internal mammary chain (7%), and spine (2%).
In this UK data series, 18 (32%) underwent a preterm delivery (<36 weeks gestation). Although complete obstetric data was missing in 37% of cases, reported delivery modalities were spontaneous vaginal delivery, assisted vaginal delivery, and caesarean section in 28%, 7%, and 33%, respectively.
In terms of regional variations, patients treated outside of London were more likely to receive radiotherapy (80% versus 65%), more likely to deliver at term (48% versus 19%), and less likely to have a caesarean (24% versus 40%). More patients received anthracycline/cyclophosphamide/taxane regimens in London (64% versus 32%), whilst the triplet regimen, with the addition of fluorouracil chemotherapy, was more commonly used outside London (13% versus 48%). Neoadjuvant chemotherapy was less frequently given in London (28% versus 56%).
CONCLUSION
Historically, uncertainties regarding the safety of treatment modalities of PABC may have led to worse outcomes in this group of younger females with breast cancer. However, consistent with more recent data, further clarity has been provided by this study on the safety of a complete, albeit adjusted, treatment pathway in this heterogeneous disease process. Although some geographical variations in the management of PABC were observed, the authors advise exercising caution in its interpretation, as these may have been impacted by year of diagnosis, stage of disease, and gestation at presentation. Further prospective work is planned to explore national variation in PABC management and patient outcomes.