Summary
Endometrial cancer is one of the most common gynaecological cancers in developed countries, and the incidence is rising significantly. The staging of this disease is evolving from anatomic staging and risk stratification to a more molecular-based stratification. Treatment of endometrial cancer is also evolving. Paclitaxel plus carboplatin is the standard first-line chemotherapy for endometrial cancer; however, there is new evidence that the combination of chemotherapy and immunotherapy has synergistic effects in the treatment of this disease. This article discusses the latest advancements in endometrial cancer research in 2023, including highlights from the Society of Gynecologic Oncology (SGO) Annual Meeting on Women’s Cancers 2023 in March, the 2023 American Society of Clinical Oncology (ASCO) Annual Meeting in June, the 24th European Gynaecological Oncology (ESGO) Congress in September and October, and the European Society for Medical Oncology (ESMO) Congress 2023 in October. The article highlights the unprecedented progression-free survival (PFS) data from two Phase III randomised controlled trials evaluating first-line immunotherapy in combination with chemotherapy in patients with advanced or recurrent endometrial cancer: RUBY with dostarlimab, and NRG-GY018 with pembrolizumab, which created a buzz at SGO 2023 in the spring, and stimulated discussion throughout the remainder of the year. The overall survival (OS) results, the clinically meaningful benefits regardless of mismatch repair status, and health-related quality of life (QOL) in these studies are also discussed. Further topics covered in this year-in-review article include the implications of the results from RUBY and NRG-GY018 on first-line treatment and recurrent settings, and the effect of adding a poly adenosine diphosphate ribose polymerase (PARP) inhibitor to immunotherapy–chemotherapy combinations in the Phase III trial, DUO-E. Disparities in endometrial cancer care, research on fertility-sparing, and the importance of the multidisciplinary team (MDT) in endometrial cancer management are also explored. Following the announcement of practice-changing findings from RUBY and NRG-GY018 in March, data presented and published throughout the remainder of 2023 show that research in endometrial cancer continues at a pace.INTRODUCTION
Endometrial cancer is one of the most common gynaecological cancers in high-income countries,1 often presents at an early stage,2 and is frequently associated with comorbidities.3 In contrast to many other cancer types, the incidence and mortality of endometrial cancer are increasing worldwide,4-7 at least in part because of the global obesity epidemic,4 with elevated mortality rates also related to the decentralisation of treatment.8,9
For many years, endometrial cancer care has been beset with poor outcomes for patients with advanced stage disease.10,11 Identifying endometrial cancer at an early stage is associated with better outcomes,1 but there is currently no public health screening programme for this type of cancer,12,13 and only females symptomatic for endometrial carcinoma (e.g., presenting with abnormal bleeding) typically seek medical help for its diagnosis and treatment.14
Disease characterisation in patients with endometrial cancer is not only a clinical process.15 The staging of endometrial cancer is evolving from anatomic staging and risk stratification to a more molecular-based stratification.16 An important development in 2023 was the publication of guidelines from the International Federation of Gynecology and Obstetrics (FIGO), in which staging in endometrial cancer now integrates molecular classification, tumour patterns, and histological staging.17 After surgery, information on which of the four molecular subtypes of endometrial cancer the patient has (polymerase ε exonuclease domain mutated, mismatch repair deficient [dMMR], p53 wild-type/copy-number-low, or p53-mutated/copy-number-high),18 in parallel with histological data, are important for FIGO staging,17 and class risk stratification. The latter guides decisions about adjuvant treatment for patients with endometrial cancer.15
Treatment for endometrial cancer is also evolving, with the development of molecular analysis and novel strategies.19 Standard first-line chemotherapy for endometrial cancer is paclitaxel plus carboplatin;20 however, the combination of chemotherapy and immunotherapy has recently gained considerable research interest in the gynaecological oncology community.20,21
Combining immunotherapy and chemotherapy has proved to be effective in the treatment of endometrial cancer, particularly dMMR disease.20,21
UNPRECEDENTED PROGRESSION-FREE SURVIVAL RESULTS FROM RUBY WITH DOSTARLIMAB AND NRG-GY018 WITH PEMBROLIZUMAB WERE A HIGHLIGHT IN 2023
Perhaps the most important highlight of endometrial cancer research in 2023 was the practice-changing results from RUBY21 with dostarlimab, and NRG-GY01820 with pembrolizumab, which were met with standing ovations and a sense of gratitude from the research community when they were announced at the SGO Annual Meeting in March 2023. Experts considered that these were two of the most striking trials ever seen in gynaecological oncology research.16
Historically, chemotherapy alone has had limited efficacy in patients with endometrial cancer.22,23 The addition of immunotherapy to chemotherapy, followed by immunotherapy maintenance, led to a marked improvement in PFS, the primary endpoint in both of these studies (Figures 1 and 2).20,21 The improvement in PFS with immunotherapy appeared to be sustained over time, as the Kaplan–Meier curves did not converge (Figures 1 and 2).20,21 Furthermore, the combination chemotherapy and immunotherapy regimens in RUBY21 and NRG-GY01820 were well tolerated, with no new safety signals.
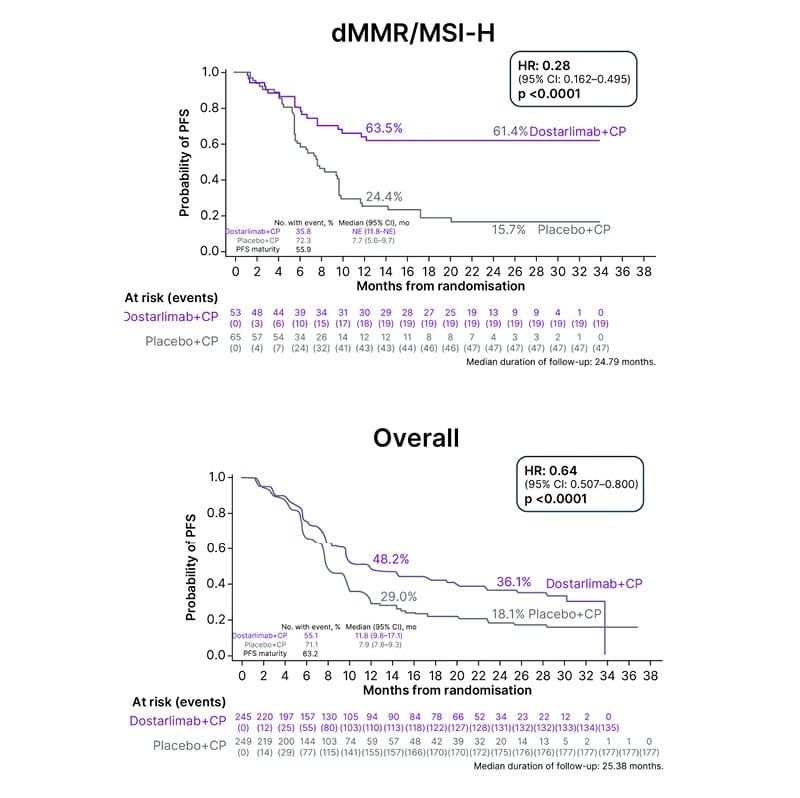
Figure 1: Statistically significant and clinically meaningful progression-free survival in RUBY.
Adapted from Mirza et al.21
CI: confidence interval; CP: carboplatin-paclitaxel; dMMR: mismatch repair deficient; HR: hazard ratio;
mo: month; MSI-H: microsatellite instability-high; NE: not evaluable; No: number; PFS: progression-free survival.
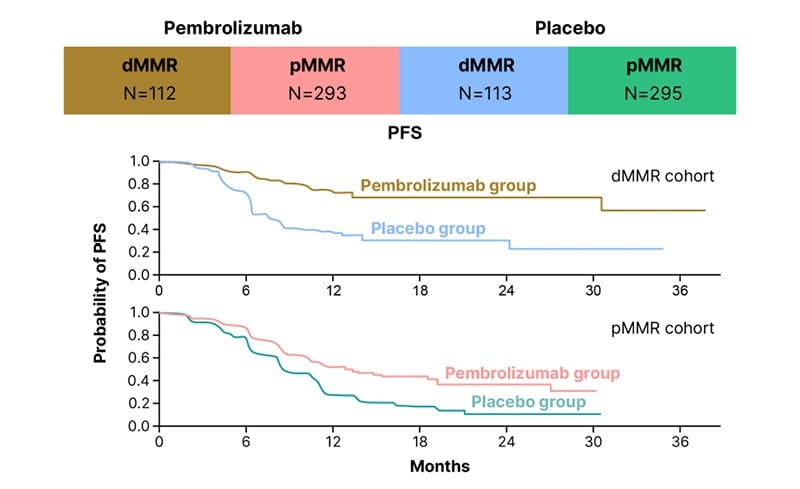
Figure 2: Significantly longer progression-free survival than with chemotherapy alone in NRG-GY018.
Adapted from Eskander et al.20
dMMR: mismatch repair deficient; PFS: progression-free survival; pMMR: mismatch repair proficient.
PROGRESSION-FREE SURVIVAL BENEFIT IN RUBY AND NRG-GY018 MAY TRANSLATE INTO AN OVERALL SURVIVAL BENEFIT
OS was a primary endpoint in RUBY,21 and a secondary endpoint in NRG-GY018.20 The OS data from these studies presented at SGO in March 2023 were not yet mature; however, there were signs from RUBY21 that the observed PFS benefit may translate into an OS benefit, with a 36% reduction in the risk of disease progression or death at 24 months in the overall population (24-month hazard ratio [HR]: 0.64; 95% confidence interval [CI]: 0.46–0.87; p=0.0021).21 From a purist standpoint, this result was not statistically significant because of the α-spin assigned to this endpoint (p=0.0021; α-spin=0.0017).16
Landmark OS with dostarlimab in RUBY21 was 71.3% (95% CI: 64.5–77.1) at 24 months in the overall population, whereas, historically, survival in patients with endometrial cancer was approximately 12 months.24
In October 2023, the primary endpoint of OS was reported to be met in RUBY.25 There was a statistically significant and clinically meaningful benefit in the overall patient population.25
CLINICALLY MEANINGFUL BENEFITS REGARDLESS OF MISMATCH REPAIR STATUS IN RUBY AND NRG-GY018
As reported at SGO 2023, clinically meaningful improvements in PFS were seen in both the dMMR and mismatch repair proficient (pMMR) populations in RUBY and NRG-GY018, with the results being more impressive in the dMMR/microsatellite instability-high (MSI-H) population in both studies.16,20,21 The dostarlimab regimen was associated with a 72% lower risk of progression or death than the placebo regimen in RUBY,21 and there was a 70% difference in relative risk for the pembrolizumab regimen versus the placebo regimen in NRG-GY018.20 The HRs for PFS in these trials were 0.28 (95% CI: 0.16–0.50; p<0.001) in RUBY,21 and 0.30 (95% CI: 0.19–0.48; p<0.001) in NRG-GY01820 in the dMMR/MSI-H populations.
The dramatic improvement in PFS in the pMMR population, shown by a 46% reduction in disease progression in NRG-GY018,20 and a 24% decrease in RUBY,21 was a surprise for many in the research community.16
In NRG-GY018,20 in the pMMR cohort, median PFS was 13.1 months with pembrolizumab versus 8.7 months with placebo (HR: 0.54; 95% CI: 0.41–0.71; p<0.001), which, although less impressive than the results for the dMMR cohort (HR: 0.30), was still a statistically significant and clinically meaningful difference.16
Notably, the Kaplan–Meier curves from these studies flattened out for both the dMMR and pMMR populations (Figures 1 and 2).16 Data from RUBY21 and NRG-GY01820 indicate a long, durable response in patients with dMMR disease.
Exploratory efficacy outcomes by molecular classification in RUBY, presented at ESMO 2023, showed clinical benefit (PFS and OS) with dostarlimab plus carboplatin–paclitaxel in dMMR/MSI-H, mutant TP53, and no specific molecular profile molecular subgroups versus placebo plus carboplatin–paclitaxel.26 These data were based on the 400 patients (of the 494 randomised) for whom mutational data were available.26
In October 2023, a clinically meaningful OS benefit was reported in both the dMMR/MSI-H and pMMR patient subgroups in RUBY.25
The clinical benefit with pembrolizumab in NRG-GY01820 was shown at ESMO 2023 to be maintained with more mature clinical follow-up in the dMMR and pMMR populations.27 Objective response rate was greater in the pembrolizumab arm than the placebo arm for both populations: 81.5% versus 70.7% (odds ratio: 1.83; 95% CI: 0.92–3.66) in the dMMR population, and 70.7% versus 58.1% (odds ratio: 1.74; 95% CI: 1.18–2.58) in the pMMR population. Furthermore, the mechanism of MMR loss did not appear to be prognostic of response to pembrolizumab.27
HEALTH-RELATED QUALITY OF LIFE IN PATIENTS WITH ENDOMETRIAL CANCER
Patient-reported outcomes were a secondary endpoint in RUBY,21 and were discussed at meetings, including ASCO28 and ESMO,29 in 2023. There were nominally significant improvements relative to baseline in patient-reported global QOL (Figure 3), and functional and symptom scales, at Cycle 7 and end of treatment, in patients with advanced or recurrent endometrial cancer, and dMMR/MSI-H tumours receiving dostarlimab plus carboplatin–paclitaxel, compared with those receiving placebo plus carboplatin–paclitaxel.29 These results, combined with the significantly improved PFS, support the use of dostarlimab plus carboplatin–paclitaxel as standard of care in this patient setting.28,29
Health-related QOL data from Study 309/KEYNOTE-77530 indicated that the tyrosine kinase inhibitor, lenvatinib, plus pembrolizumab, had an overall favourable benefit/risk profile versus treatment of physician’s choice for patients with advanced endometrial cancer.31 Coupled with the efficacy and safety data from this study,30 the patient-reported outcome data support the use of lenvatinib plus pembrolizumab in endometrial cancer.31
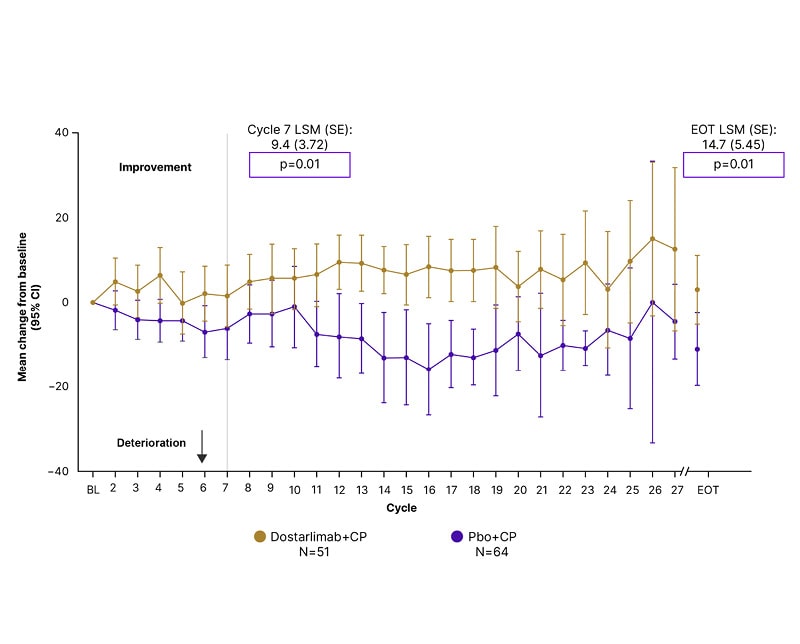
Figure 3: Global quality of life in the mismatch repair deficient/microsatellite instability-high population in RUBY.*
*Mixed models for repeated measures analysis was conducted to generate least square means, and to determine the difference between trial arms for patient-reported outcome assessment change from baseline at individual time points. Mixed models for repeated measures analysis was not adjusted for multiple testing/multiplicity; therefore, p-values were nominal.
CI: confidence interval; CP: carboplatin+paclitaxel; EOT: end of treatment; LSM: least square mean; Pbo+CP: placebo+carboplatin; SE: standard error.
Impact of the Results of RUBY and NRG-GY018 on First-Line Treatment of Endometrial Cancer
The impressive results from RUBY21 and NRG-GY018,20 presented at SGO 2023, led to a change in the standard of care first-line treatment for endometrial cancer in 2023.16,32-35 In a follow-up32 to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology for Uterine Neoplasms Version 1.2023,36 panel consensus (based on RUBY21 and NRG-GY01820) was to include: carboplatin/paclitaxel/dostarlimab as a primary or adjuvant treatment option for Stage III–IV endometrial carcinoma; carboplatin/paclitaxel/dostarlimab as a first-line treatment option for recurrent endometrial carcinoma; carboplatin/paclitaxel/pembrolizumab as a primary or adjuvant treatment option for Stage III–IV endometrial carcinoma (except for carcinosarcoma); and carboplatin/paclitaxel/pembrolizumab as a first-line treatment option for recurrent endometrial carcinoma (except for carcinosarcoma).32
Although many physicians changed treatment practices immediately after the presentation of the results of RUBY21 and NRG-GY01820 at SGO 2023, controversially, a few institutions did not accept the idea of adding immunotherapy to chemotherapy before adjuvant chemotherapy, because the OS benefit in RUBY21 was not technically statistically significant.16 However, most of the community interpreted these data as indicating a pronounced improvement in the first-line setting.16
Dostarlimab plus chemotherapy in the first-line for patients with primary advanced or recurrent endometrial cancer with dMMR/MSI-H tumours received U.S. Food and Drug Administration (FDA) approval on 31st July 2023;33 UK Medicines and Healthcare products Regulatory Agency (MHRA) authorisation on 2nd October 2023;34 and approval in the European Union on 11th December 2023,35 with use in Europe determined by local guidelines.16
Implications of the Results of RUBY and NRG-GY018 on Second-Line Therapy in Patients with Endometrial Cancer
The impact of the results of RUBY21 and NRG-GY018,20 presented in March 2023, on second-line care in patients with endometrial cancer was not immediately clear, because frontline data changed practice, and there were no data on the use of immunotherapy in the recurrent setting after first-line immunotherapy in endometrial cancer. The standard treatment option for patients with pMMR tumours is a combination of lenvatinib with pembrolizumab, which is an approved second-line therapy.37 Second-line therapy for patients with dMMR tumours was a more difficult decision; however, the hope was that the majority of these patients would not have disease recurrence.16 In patients with disease recurrence, treatment options might include utilising the same immunotherapy as used in first-line (with or without chemotherapy), or administering other immunotherapy regimens, such as lenvatinib–pembrolizumab, or immunotherapy plus a vascular endothelial growth factor inhibitor; however, there is a lack of clinical study data to guide these treatment decisions.16 Potential options for second-line treatment of dMMR/MSI-H advanced or recurrent endometrial cancer were expanded in 2023, with the regular approval of dostarlimab by the FDA on 9th February, and the full approval for dostarlimab as a monotherapy granted by the European Commission on 11th December.35
Effect of Adding a PARP Inhibitor to Immunotherapy-Chemotherapy Combination in DUO-E
Adding a PARP inhibitor (e.g., olaparib) to immunotherapy–chemotherapy combinations may improve outcomes.38 DUO-E was a Phase III study of 718 patients with newly diagnosed advanced or recurrent endometrial cancer, randomised in a 1:1:1 ratio to control arm (durvalumab placebo plus carboplatin and paclitaxel, then durvalumab placebo plus olaparib placebo); durvalumab arm (durvalumab plus carboplatin and paclitaxel, then durvalumab plus olaparib placebo), or durvalumab plus olaparib arm (durvalumab plus carboplatin and paclitaxel, then durvalumab plus olaparib).39,40
There was statistically significant and clinically meaningful improvement in PFS for the durvalumab (HR: 0.71; 95% CI: 0.57–0.89; p=0.003) and durvalumab plus olaparib (HR: 0.55; 95% CI: 0.43–0.69; p<0.0001) arms versus control. Prespecified, exploratory subgroup analyses showed PFS benefit in the dMMR subgroup for the durvalumab (HR: 0.42; 95% CI: 0.22–0.80) and durvalumab plus olaparib (HR: 0.41; 95% CI: 0.21–0.75) arms versus control.39,40 Adding olaparib to durvalumab also enhanced PFS benefit in the pMMR subgroup, with an HR of 0.57 (95% CI: 0.44–0.73) for durvalumab plus olaparib versus control compared with 0.77 (95% CI: 0.60–0.97) for durvalumab versus control.39,40
According to Lorusso and Monk,41 the results of DUO-E are difficult to interpret, because the study design does not distinguish the relative contribution of PARP inhibitor to treatment outcome. Indeed, Monk was sceptical that PARP inhibitors would be accepted in this scenario from a regulatory standpoint, because of the challenges in study design.41
OTHER KEY PHASE III CLINICAL STUDIES IN ENDOMETRIAL CANCER PRESENTED AT CONGRESSES IN 2023
A study of particular interest at SGO 2023 was NRG258.16,42 This was a randomised trial of chemotherapy and radiation compared to radiation alone in patients with Stage III/IVa endometrial cancer with <2 cm of residual tumour, or Stage I/II clear cell or serous cancer with positive cytology.42 The stratified HR for death for chemotherapy plus radiation versus radiation alone was 1.05 (95% CI: 0.82–1.34; p=0.72).42 There were no subgroups (age, BMI, stage, residual disease) that predicted an OS benefit from the combined chemotherapy and radiation treatment.42
Ongoing clinical benefit in patients with advanced endometrial cancer who had received prior platinum therapy, and who completed 2 years of pembrolizumab treatment and continued lenvatinib in Study 309/KEYNOTE-775,30 was reported at ESMO 2023.43 Median PFS in months (95% CI) was 34.1 (20.1, not evaluable) in patients with pMMR, and 34.1 (27.7, not evaluable) in the overall patient population.43 These results support lenvatinib plus pembrolizumab as standard of care in these patients.43
Also presented at ESMO 2023, the addition of the programmed death-ligand 1 inhibitor atezolizumab to standard carboplatin–paclitaxel chemotherapy in AtTEnd was associated with a statistically significant improvement in PFS for patients with advanced or recurrent endometrial carcinoma (HR: 0.74; 95% CI: 0.61–0.91; p=0.0219), with a substantial benefit observed in patients with dMMR carcinomas (HR: 0.36; 95% CI: 0.23–0.57; p=0.0005).44
Immunotherapy Versus Chemotherapy in the First-Line Setting in Patients with Endometrial Cancer
KEYNOTE-C93,45 DOMENICA,46,47 and LEAP-00148 are ongoing studies to compare immunotherapy versus chemotherapy in the first-line setting in patients with advanced or recurrent endometrial cancer. Although these studies do not have the new standard of care (i.e., chemotherapy plus immunotherapy) as a control arm, and thus may be difficult to interpret, they are still considered important.16 The data on immunotherapy alone versus chemotherapy alone that these studies will provide may help to guide next steps in research, and potentially increase treatment choice, perhaps including non-chemotherapy-based treatment, for patients.16 Future studies may comprise chemotherapy plus immunotherapy versus immunotherapy alone, but are unlikely to include chemotherapy alone, as adding immunotherapy to chemotherapy greatly improves response.16
DISPARITIES IN ENDOMETRIAL CANCER CARE
Disparities in the incidence and outcome of gynaecological cancers are complex and multifactorial.49 Barriers to endometrial cancer care include lack of knowledge on endometrial cancer; poor communication; and clinical, administrative, financial, geographical, and facility-related difficulties.50 Disparities in endometrial cancer care are gaining increasing interest in the oncology community as part of the continuous drive to improve patient outcomes,51 and were a topic of discussion in 2023.41,51-53 Disparities in patient management, and outcomes among patients with endometrial cancer, arise from racial, socioeconomic, educational, and geographical barriers, which influence treatment and survival.51 A further concern surrounding endometrial cancer is the disparities in clinical trial involvement.54-56 Differences in access to treatment, and adherence to treatment guidelines, including inequalities in surgical care, adjuvant chemotherapy and radiation treatment, and socioeconomic status, have been shown in numerous studies in the USA, but there are few treatment disparities in Europe, where therapy is easier to access for all populations.51 There are higher rates of more aggressive endometrial cancer in Black patients compared with White patients.57 Furthermore, biological factors, such as comorbidity, are an important cause of disparity among females with endometrial cancer.51 The higher incidence of high-risk endometrial cancer in Black compared with White females has not been adequately researched, and further work is needed to improve treatment outcomes for Black females with low socioeconomic status.51 To understand and address these disparities requires further research, in which diverse populations are represented.16 In addition, education about endometrial cancer, healthy lifestyle, and preventative strategies to decrease comorbidity rates will help to reduce the disparity between Black and White patients, and between patients with low and high socioeconomic status.51
Fertility-Sparing Management of Young Females with Endometrial Cancer
Primary surgery is effective in low-risk endometrial cancer; however, this approach compromises fertility in young females.58 Fertility-sparing management, such as progestin-based therapy, can be considered in cases of atypical endometrial hyperplasia, or Grade 1 endometrial cancer, in females of reproductive age.58-61 The levonorgestrel intrauterine device is often utilised in these patients.62 There is an unmet need for a personalised treatment approach in cases of first-line progestin treatment failure.62 In this context, there is growing interest in glucagon-like peptide 1 receptor agonists for fertility-sparing in patients with malignant endometrial pathology who are obese, and this area warrants further exploration.41,62
ESGO, the European Society of Human Reproduction and Embryology (ESHRE), and the European Society for Gynaecological Endoscopy (ESGE) collaboratively published guidelines in 2023 for the fertility-sparing treatment of patients with endometrial carcinoma; however, the majority of the recommendations were supported only by observational data, professional experience, and consensus of the development group, rather than high-level, scientific evidence.63
Importance of the Multidisciplinary Team in Endometrial Cancer Care
The recent increase in the understanding of tumour biology in endometrial cancer, as well as a significant improvement in tailoring surgery and radiotherapy, and the introduction of targeted therapies, prompt the need for novel initiatives to increase the awareness of new treatment modalities.15 Healthcare infrastructure is required to enable the optimal management of patients with endometrial cancer, particularly considering the increasing complexity of the medical and surgical treatment of this disease.15 A co-ordinated, collaborative, multidisciplinary approach from healthcare professionals from different specialisms, incorporating evidence-based guidelines,7 quality indicators,64 and shared decision-making, is necessary for the management of patients with endometrial cancer, to diagnose and treat the disease, provide support for the various needs of the patients, and optimise outcomes.15,65
An MDT approach is considered the gold standard for the diagnosis and treatment of cancer, and is an evolving area of oncology.66-68 Although an MDT approach to support patients with endometrial cancer is becoming increasingly common in clinical practice across the oncology community, with recent examples in the management of metastatic disease and relapse,69 increasing discussion about obesity and weight loss,70 and preserving fertility,71 it is not yet a global approach.15
A multidisciplinary approach should be adopted globally to provide optimum care for patients with endometrial cancer; however, the process of forming an MDT is not the endpoint.15 Optimal management of patients with endometrial cancer requires healthcare professionals to be trained and incentivised to work together effectively in a multidisciplinary context.15
FUTURE PROSPECTS AND CONCLUSIONS
Following the presentation of practice-changing findings from RUBY21 and NRG-GY01820 in patients with advanced or recurrent endometrial cancer at the SGO Annual Meeting in March 2023, data presented and published throughout the remainder of 2023 show that research in endometrial cancer continues at a pace.
Observations in 2023 of marked and sustained improvement in PFS, clinically meaningful OS benefit, maintained clinical benefit with more mature clinical follow-up, improved QOL, and efficacy of new treatment combinations, are important steps towards improved outcomes in patients with endometrial cancer. The exceptional results from RUBY21 and NRG-GY01820 led to a change in the standard of care first-line treatment for endometrial cancer. However, second-line treatment of endometrial cancer is an unmet medical need.72 Research is needed to define and optimise second-line regimens for patients with dMMR and disease recurrence, and to optimise treatment in patients with pMMR, to enable treatment responses similar to those seen in patients with dMMR. A deeper understanding of potential mechanisms of resistance to immunotherapy in pMMR endometrial cancer is needed for the development of novel therapeutic approaches.73
Further research into the use of immunotherapy, including combination with other agents, in light of the considerable clinical benefit shown in RUBY21 and NRG-GY018;20 and efforts towards personalised treatment for patients with rare histological subtypes, such as clear cell or malignant mixed Müllerian tumour (also known as carcinosarcoma) and specific molecular profiles, are also important in the endometrial cancer field. In addition, following the results of DUO-E, research is needed into the potential role of PARP inhibitors in endometrial cancer treatment. The potential for chemotherapy-free treatment remains a topic of research, and the results from KEYNOTE-C93,45 DOMENICA,46,47 and LEAP-00148 are awaited with interest by the gynaecological oncology community.
Global efforts are needed to reduce disparities in endometrial cancer care, including clinical studies in which diverse populations are represented, and education about endometrial cancer and preventative strategies to decrease comorbidity. Furthermore, it is essential that all patients with endometrial cancer have access to therapies. Additional research into fertility-sparing techniques, and the global adoption of an MDT approach in endometrial cancer care are further considerations to improve patient outcomes.
The complexity of endometrial cancer has recently become evident, with the discovery of four different types of tumour, and this has changed the research and treatment landscape for this type of cancer. Clinical research in endometrial cancer in 2024 and beyond will build on the considerable progress made in 2023, as part of a continuous drive to improve patient outcomes.







