Abstract
Introduction: Accurate prediction of recurrence is important for patients with non-muscle invasive bladder cancer (NMIBC).
Objective: To study the association of tumour location with recurrence-free survival (RFS) of patients with primary solitary tumours.
Methods: Patients (N=184) with primary, solitary NMIBC (2000–2018). In cases of overlapping areas, the most involved area was selected. Subsequently, the areas were dichotomised into dorsal versus non-dorsal tumours. The dorsal area was defined as the diamond-shaped area bordered by bladder neck, trigone, posterior wall, and orifices. The non-dorsal areas are the lateral walls, dome, and anterior wall. The association of location with RFS was assessed using Cox regression. Median RFS was estimated using the Kaplan–Meier method.
Results: Altogether, 25 (14%) and 69 (38%) patients had a recurrence at 1 year and 5 years, respectively. Median RFS was 103 months. Primary tumours located at the anterior wall were associated with the lowest RFS (median: 74 months) and at the posterior wall with highest RFS (median: 133 months). After dichotomisation, 54% of the patients had a tumour in the dorsal area with a median 1-year recurrence rate of 9% versus 19% in the non-dorsal area. Median RFS in the dorsal area was 133 months versus 48 months in the non-dorsal area (p=0.02). Cox analysis showed worse 1- and 5-year RFS on adjusted analysis (hazard ratio [HR]: 2.41; 95% confidence interval [CI]: 1.07–5.46; p=0.04; and HR: 1.77; 95% CI: 1.10–2.85; p=0.02, respectively) for tumours in the non-dorsal area.
Conclusion: The tumours in the dorsal area appear to have a lower recurrence rate. There was no association with specific tumour location and RFS.
INTRODUCTION
Risk stratification and prognosis estimation is important in patients with non-muscle invasive bladder cancer (NMIBC). NMIBC has a high probability of recurrence and, to a lesser extent, progression into muscle invasive disease at a later stage.1 Long-term recurrence rates as high as 80% have been reported,2 and up to 45% of tumours progress.3 The need for surveillance for tumour recurrence and treatment complications results in high lifetime treatment costs, making bladder cancer the most expensive cancer.4
Because treatment of NMIBC is based on prognosis, several prediction systems have been developed to predict short- and long-term risks. The risk tables of the European Organisation for Research and Treatment of Cancer (EORTC) and the scoring model of the Spanish Urological Club for Oncological Treatment (CUETO) are two prediction systems that are currently recommended by guidelines internationally.1,5 Both systems assess the probability of 1-year and 5-year recurrence and progression based on a combination of clinical and pathological factors.3,6 Some of the included factors can be regarded as subjective and are operator dependent, i.e., determination of the number of tumours and estimation of tumour size during a transurethral resection (TURBT).7 The assessment of tumour stage and histological grade is also associated with high interobserver variability.8,9 Moreover, the prognostic value of these prediction models are limited because the populations on which the models are based were treated differently than recommended by the current standard.10
Tumour location is not considered in risk assessments of patients with NMIBC. However, Palou et al.11 have shown that tumours in the trigonal area are associated with a higher probability of upper urinary tract tumour (UTUC) presence. Indeed, as is mentioned in the guidelines, imaging of the upper tract has to be considered when finding a tumour in the trigone.1,5 However, the association of tumour location with recurrence-free survival (RFS) has only been studied in a limited number of patients in heterogeneous datasets, with the inclusion of patients with NMIBC and muscle invasive disease or those with multiple tumour locations and/or used both of their primary and recurrent tumours.12–14 To improve the understanding of the association of intravesical tumour location with RFS, the disease outcome of patients with primary solitary bladder tumours was assessed. The aim of this study was to identify the influence of location of the urothelial cell carcinoma of the bladder on 1- and 5-year RFS.
METHODS
Data Acquisition
This study was approved by the medical ethical committee of the Amsterdam UMC, location AMC (W17_327_ # 17.380). A database was constructed using the Data Management System (v 3.1.3, T&S innovations, Utrecht, the Netherlands). All patient data of the 840 patients who underwent TURBT between 2000–2018 were retrospectively collected. Patients were given the possibility to opt-out from the study following the Dutch Act on the Protection of Personal Data and Code Good Conduct.
Only patients with primary, solitary, NMIBC urothelial cell carcinoma that were radically resected during the primary TURBT or re-TURBT and did not contain concomitant carcinoma in situ were included. If a tumour was upstaged to muscle invasive bladder cancer during the re-TURBT, the patient was excluded. Additionally, patients with a prior or simultaneous UTUC and patients who underwent a radical cystectomy after the first TURBT were excluded from the study. Follow-up was performed according to the European Association of Urology (EAU) guidelines, with the first follow up cystoscopy after 3 months. Therefore, patients with a follow-up period of <3 months were also excluded.
Tumour locations were assessed by retrospectively checking the reports of the initial cystoscopy, operation, and pathology. Locations were characterised using the bladder map of the EAU guideline, with the exception that trigone and bladder neck were grouped together. Tumours located in the urethra were excluded from the study because it was not possible to assess the exact location (pre- or post- sphincteric).
Besides assessing the specific tumour locations, areas were grouped into dorsal versus non-dorsal tumours. The dorsal area was defined as the diamond-shaped area bordered by the bladder neck, trigone, posterior wall, and orifices. The non-dorsal areas are the dome, anterior wall, and lateral walls. In case of a large tumour spreading out over multiple regions, the most involved area was selected.
Covariates
The association of location with outcome variables was adjusted for patient and tumour specific characteristics. Patient characteristics included age at diagnosis, sex, postoperative bladder rinses, and adjuvant treatment (intravesical chemotherapy/immunotherapy). Tumour specific characteristics included T-stage (based on the pathology report), Grade (based on both the World Health Organization [WHO]’73 and WHO’04 grading system) and tumour size (<3 and >3 cm). Recurrence was defined as a pathologically proven recurrence.
Outcomes
The outcomes were compared for tumour location specific and dorsal versus non-dorsal area. The primary outcome was defined as RFS. Progression was defined as pathologically proven tumours invading the muscularis propria, imaging-proven nodal or distant metastasis, or UTUC.
Statistical Analysis
Kaplan–Meier survival analysis and log-rank test were used for RFS estimates between groups. Patients without recurrence were censored at last follow-up and patients with incomplete follow-up were censored at the last date of observation. Groups were compared using Pearson’s chi-square test. The Cox proportional hazard model was used to assess the association of location with 1- and 5-year RFS. Multicollinearity was measured by variance inflation factors. Variables with a statistically significant association with the outcome measures in the unadjusted analyses were considered in the adjusted analysis. These results were only reported when the association was confirmed. Statistical significance was considered at p<0.05. All statistical tests were performed using SPSS® (IBM, New York City, New York, USA) (v25).
RESULTS
Baseline Characteristics
A total of 184 patients were included into the analysis with a median age of 67 years (interquartile range [IQR]: 57–74) and 24% (n=44) were female. Tumour stage was Ta in 81% (n=149) and T1 in 10% (n=19) patients. Data of both the WHO’73 and WHO’04 grading systems were collected. WHO’73 grading was divided as follows: Grade 1 was 16% (n=30), Grade 2 was 52% (n=96), and Grade 3 was 29% (n=53). In 3% (n=5) of patients the WHO’73 grade was unknown. The WHO’04 grade was unknown in 33% (n=61) of patients, and 17% (n=31) had a low-grade tumour and 36% (n=66) had a high-grade tumour. For the tumour size, the definition according to the EAU risk stratification was used. In 71% (n=130) of patients the tumour was <3 cm and in 23% (n=43) the tumour was >3 cm. In 6% (n=11) of cases the tumour size was unknown. In 103 (56%) patients postoperative mitomycin (MMC) was given. Adjuvant therapy in the form of MMC was given to 5% (n=10) of patients and 17% (n=32) received adjuvant Bacillus Calmette–Guérin (BCG). The most common tumour locations were the lateral walls (45%, n=82). The median time of follow-up was 68 months (IQR: 39–115). In the total cohort, 25 (14%) and 69 (38%) patients had a recurrence at 1 year and 5 years, respectively. Progression was seen in two (1.1%) and six (3.3%) patients at 1 and 5 years, respectively.
Recurrence-Free Survival Per Tumour Site
In 12 patients the primary tumour location could not be assessed, and they were excluded from the analysis for specific tumour location. Recurrence rates at 1 and 5 years are shown in Table 1. Recurrence rates at 1 year ranged from 0% for patients with a primary tumour within the posterior wall to 50% for primary tumours within the anterior wall (p=0.22). Recurrence rates at 5 years ranged from 20% for patients with a primary tumour within the posterior wall to 50% for patients with a primary tumour within the anterior wall (p=0.67).
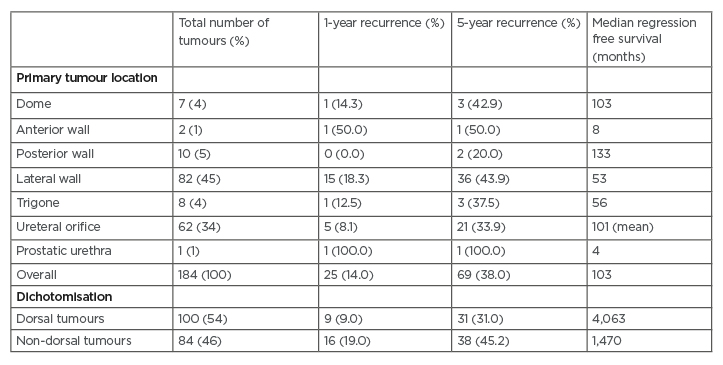
Table 1: Overview of recurrence rates and median recurrence-free survival for specific tumour locations and dorsal versus non-dorsal tumours (in number of tumours and percentage of recurrence at each location).
In case of overlapping tumour areas, only the main tumour location was taken into account. Proportions may not total 100% as a result of unknown main tumour area.
Median RFS was 103 months (Table 1). Median RFS of specific tumour locations ranged from 8 months for tumours at the anterior wall to 133 months for tumours at the posterior wall. Because of the small number of events per tumour location, Cox regression could not be performed.
According to the Kaplan–Meier analysis, specific tumour locations were not statistically significant to be associated with 1- and 5-year RFS (log-rank: p=0.19; log-rank: p=0.43, respectively). No statistically significant differences were seen in recurrence rate and RFS among different adjuvant treatment groups.
Because of the small population, comparison of progression rate per tumour site was not feasible. However, 5-year progression rate was highest in the trigone (25%), made up of only 2/8 patients.
Dorsal Versus Non-Dorsal Area
Tumours in the dorsal area were seen in 54% (n=100) of patients and in the non-dorsal area in 46% (n=84) of patients, of which 9% and 19%, respectively, had a recurrence within 1 year (p<0.05). Recurrence rates at 5-years were 31% and 45% in the dorsal and non-dorsal area, respectively (p<0.05). No differences were observed in tumour grade (WHO’73: p=0.31; WHO’04: p=0.22) and T-stage (p=0.70) between the two groups.
Median RFS was 133 months for patients with a tumour located in the dorsal area, and 48 months for patients with tumours in the non-dorsal area (Figure 1) (1- and 5-year RFS, log-rank: p=0.02; log-rank: p=0.03, respectively). The WHO’04 and WHO’73 showed multicollinearity (variance inflation factor: 3.56), therefore only the WHO’04 grade was used in the analysis. On unadjusted analysis, T-stage (HR: 2.68; 95% CI: 1.18–6.07; p=0.02) and WHO’04 grade (HR: 8.60; 95% CI: 1.99–37.22; p<0.01) were statistically significantly associated with 1-year RFS. Age (HR: 1.02; 95% CI: 1.00–1.04; p=0.03), T-stage (HR: 1.73; 95% CI: 1.01–2.96; p<0.05), and WHO’04 grade (HR: 3.31; 95% CI: 1.17–6.39; p<0.01) were statistically significantly associated with 5-year RFS. Tumour location within the non-dorsal area was statistically significantly associated with worse 1- and 5-year RFS on unadjusted analysis (HR: 2.36; 95% CI: 1.04–5.33; p=0.04 and HR: 1.74; 95% CI: 1.08–2.80, respectively; Table 2).
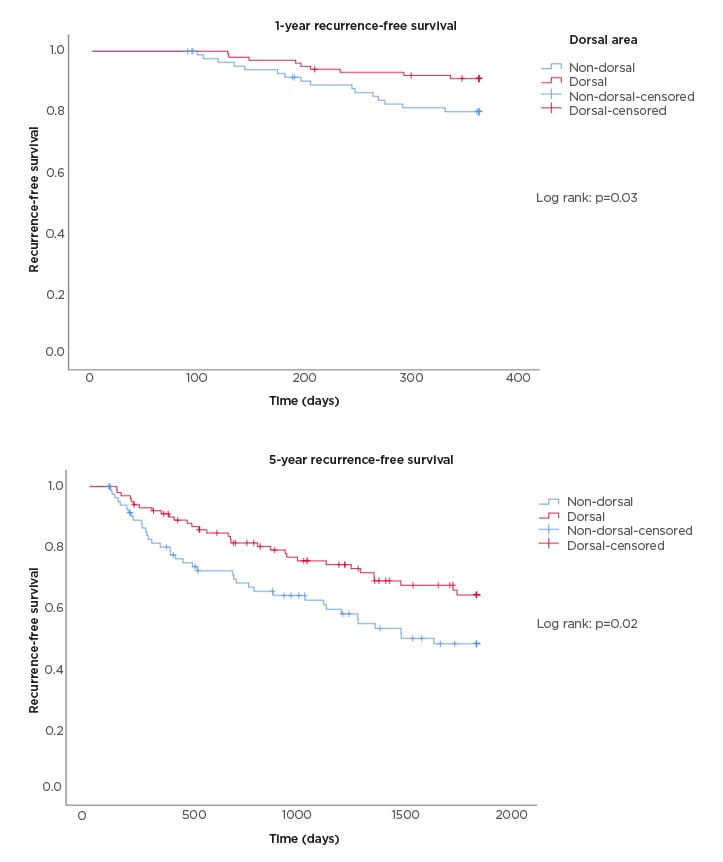
Figure 1: Kaplan–Meier curves for 1-year (top panel) and 5-year (bottom panel) recurrence-free survival based on dorsal and non-dorsal tumour area.
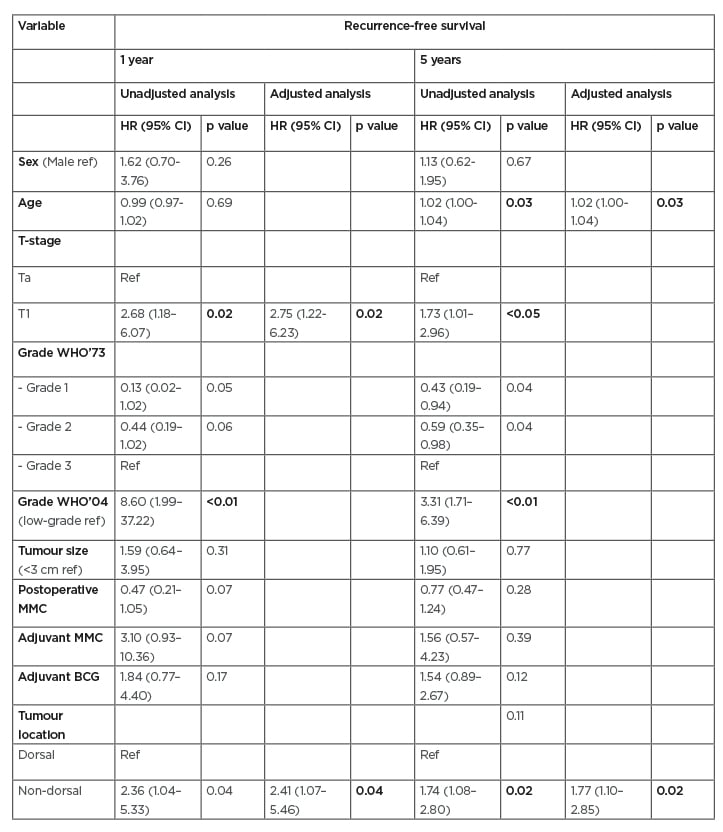
Table 2: Cox regression (unadjusted and adjusted analysis) for recurrence-free survival based on tumours in the non-dorsal area.
BCG: Bacillus Calmette–Guérin; CI: confidence interval; HR: hazard ratio; MMC: mitomycin; ref: reference; WHO: World Health Organization.
The adjusted analysis showed that only the dorsal versus non-dorsal area was significantly associated with a shorter 1- and 5-year RFS (HR: 2.41; 95% CI: 1.07–5.46; p=0.04 and HR: 1.77; 95% CI: 1.10–2.85; p=0.02; Table 2).
In the dorsal area, 1- and 5-year progression was observed in 1% and 3% of the patients, respectively. For the non-dorsal area, progression incidences were 1% and 4%, respectively. Four patients developed an UTUC during follow-up, of which two patients had an initial tumour in the dorsal area and two in the non-dorsal area.
DISCUSSION
This study assessed the association of intravesical tumour location with RFS. The main findings are that tumours located in the non-dorsal area of the bladder were associated with shorter RFS. However, no significant association of more specific tumour locations with RFS was found in this study.
As the recurrence rate of NMIBC is a relevant parameter for the determination of the need and options of adjuvant treatment, several risk prediction systems have been constructed.3,6 In these nomograms, different clinical and pathological parameters related to recurrence rates are assessed. The primary location of the tumour is not a parameter in these prediction systems. Over time, only three studies have studied the association of tumour location with recurrence.12,15,16 Mulders et al.15 prospectively studied 371 patients with NMIBC. They identified bladder neck, prostatic urethra, posterior wall, and trigone separately as regions associated with a shorter recurrence-free interval. Vukomanovic et al.16 studied a group of 74 patients with T1 high-grade NMIBC, which they divided into patients treated with TURBT and BCG versus TURBT alone. For patients treated with BCG, recurrence was more common when having a tumour in the bladder neck, whereas in patients treated with TURBT alone, tumours in the lateral walls and orifices were associated with recurrence. Segal et al.12 analysed a group of 278 patients with T1 high-grade NMIBC and found that tumours located in the trigone were associated with shorter RFS on adjusted analysis. The main weakness of these three studies is the low number of events per tumour location, making the statistical models unreliable.17 Similarly, the dataset was too small to assess the association of specific tumour location with RFS. However, this study found a higher recurrence rate and a shorter RFS in patients with a tumour in the non-dorsal area in contrast to the previous studies.12,15,16
Only a small number of studies have studied the influence of intravesical tumour location on progression.18,19 Kobayashi et al.18 found, in a population of 297 patients with NMIBC, tumours within bladder neck to be significantly associated with progression, whereas Weiner et al.19 showed that tumours in the dome were statistically significant and associated with advanced stage at the time of radical cystectomy. The sample size of this study was unfortunately insufficient to assess the association of tumour location with progression.
To the authors’ knowledge, this is the first study to determine the association of intravesical tumour location with RFS, including only patients with primary, solitary NMIBC. Most studies that considered the relation of tumour location with recurrence have used heterogeneous datasets, including recurrent and multiple tumours. However, both variables have been significantly associated with lower RFS.3 In comparison with the EORTC, this study population did receive postoperative MMC, or adjuvant BCG or MMC if indicated. The main weakness of this study is the retrospective character, which may contribute to a lack of standardisation and tumour location description. Although adequate documentation of tumour location by using a bladder diagram has proven to reduce the recurrence rates,20 in this dataset several patients were excluded due to a missing description of tumour location. Another variable that should have been taken into account is the surgical experience of the surgeon performing the TURBT. A TURBT performed by an experienced surgeon has shown to decrease the recurrence rate.21,22 The surgical reports did not clearly state the exact role and amount of supervision when a resident was present during the TURBT, therefore this variable was not included into the analysis. Moreover, the different molecular subtypes of bladder cancer were not accounted for. As for muscle invasive bladder cancer, NMIBC has comparable subtypes which influences outcome.23 Finally, due to the limited sample size, the number of events per variable was limited. Therefore, performing a Cox regression analysis on specific tumour locations would have induced an overestimation of the significance.17 Because the number of patients was limited, the number of variables that could be taken into account in the multivariable analysis was also limited. Therefore, this study may not be enough to present final conclusions. Dichotomisation of the data made it possible to assess the HR of the dorsal and non-dorsal tumours. Because the trigonal area is often difficult to define, the bladder neck, trigone, orifices, and dorsal area were grouped together.
The current risk stratification models use variables that are highly susceptible for interobserver variation and are unable to correctly predict recurrence rates.10 Consequently, external validations demonstrated low concordence-indices.24 Therefore, the search for better predictors is ongoing. However, since the areas within the bladder are also susceptible for interobserver variation, the use of the intravesical tumour location as a suitable characteristic for risk stratification is also debatable. Because the bladder is a spherical organ, areas within the bladder are hard to define.
Several mechanisms of recurrence have been described.13 Tumour seeding is a well-known mechanism, where tumour cell implantation occurs after trauma of the urothelial layer following thermal or mechanic injury.25,26 This knowledge has led to the introduction of instillation of postoperative chemotherapy to induce tumour cell lysis.14 Field cancerisation may imply that micro tumours already exist during primary TURBT. Since the whole bladder is exposed to the same carcinogens, genetically unrelated tumours arise in different parts of the bladder.27 As a result, new tumour formation is also scored as recurrence.
Several reasons for the possible relation of the non-dorsal tumour location and recurrence can be hypothesised. Most likely, inadequate resection plays an important role in the shorter RFS. Tumours in the non-dorsal area are somewhat more difficult to assess during cystoscopy, complicating radical TURBT. Moreover, during TURBT a rigid cystoscope is preferably used, which ensures less visibility of the non-dorsal side compared to a flexible cystoscope. This may cause incomplete TURBT. To overcome this problem, additional techniques might be considered to evaluate adequate resection, such as narrow band imaging28 or photodynamic diagnosis.29 Other mechanisms of action, for example those related to the flow of urine, which induces differences in contact time of the bladder wall areas with carcinogenic substances within urine, may also play a role in the recurrence rates of these tumours.
Risk stratification is important to enable comparison of outcomes and standardisation of treatment and follow-up. However, external validation of the EORTC and CUETO shows low concordance-indices for both risk tools.10 A possible explanation is that both tools are based on research data of >20 years ago and does not reflect the current standards of treatment. Therefore, it was hypothesised that tumour location within the bladder could be an additional parameter. These results may imply that TURBT techniques for a tumour in the non-dorsal area needs to be improved and an active follow-up is required for tumours in this area. A prospective study is needed to deliver a more powerful analysis that would be able to give an update of the existing risk stratification models.
CONCLUSION
This study has demonstrated that a primary tumour within the non-dorsal area is significantly associated with a shorter 1- and 5-year RFS. A significant association of more specific tumour locations with RFS was not found. The findings warrant further, preferably prospective, investigation into the role of intravesical tumour location on the outcome of patients with NMIBC.







