Abstract
Therapeutic strategies for the treatment of breast cancer have historically been determined by the presence or absence of hormone receptors and HER2 amplification and/or protein expression. For patients with breast cancer that lack these biomarkers, the so-called ‘triple-negative’ subtype, chemotherapy has been the cornerstone of cure and palliation. However, with the recent successful development of immune checkpoint molecules that target cytotoxic T-lymphocyte antigen-4, programmed cell death-1 (PD-1), and PD-ligand 1 (PD-L1), improved survival has been reported across a range of tumour types including melanoma, lung, and bladder cancer. In metastatic breast cancer, trials of single-agent immune checkpoint inhibitors (ICI) have resulted in limited overall response rates; however, strategies that combine local or systemic therapies with ICI have improved response rates and, in some cases, improved survival. For example, the addition of an anti-PD-L1 inhibitor, atezolizumab, to nab-paclitaxel chemotherapy for newly diagnosed metastatic triple-negative breast cancer demonstrated an improvement in overall survival in an informal analysis of the PD-L1-positive subset in a recently reported Phase III clinical trial. These results ultimately led to U.S. Food and Drug Administration (FDA) approval for an ICI for the treatment of breast cancer, with numerous other health authorities following suit. Herein, the authors describe the biology behind ICI, the rationale for ICI administration in breast cancer, the related clinical trial data reported to date, and promising future strategies.
INTRODUCTION
The complexity behind the treatment of breast cancer arises, in part, from the presence of biologically distinct subtypes. Clinically, subtypes are currently defined either by immunohistochemistry for hormone receptor and HER2 status,1,2 or by high-throughput diagnostic technology such as gene expression arrays or next-generation sequencing.3 From third-generation chemotherapy to monoclonal antibodies and tyrosine kinase inhibitors, the breast cancer treatment armamentarium continues to evolve.4 However, innovative strategies are still needed to improve cure rates.
Integrating systemic and local therapy has been shown to be the best strategy to achieve long-term survival for early-stage breast cancer.5 In the metastatic breast cancer (MBC) scenario, symptom control and increased survival are the most important objectives during systemic therapy. For patients with oestrogen receptor (ER) positive (+) breast cancer, great advances have been achieved in recent years incorporating targeted therapies such as mTOR inhibitors, cyclin-dependent kinase 4/6 inhibitors, and phosphoinositide 3-kinase inhibitors with conventional hormone therapy.6 Treatment of HER2+ MBC dramatically changed with dual HER2 blockade and trastuzumab emtansine treatment.7,8 In patients with BRCA1/2 germline mutations, treatment with poly (ADP-ribose) polymerase (PARP) inhibitors increases survival, compared with conventional chemotherapy.9 Patients lacking ER, HER2, and progesterone receptor expression have the triple-negative breast cancer (TNBC) subtype. Unfortunately, patients with TNBC with no BRCA mutations only have chemotherapy as an available therapy option, and this treatment generally has a poor prognosis.10
Tumour-infiltrating lymphocytes (TIL) are mononuclear immune cells that infiltrate tumour tissue and have been observed in most types of solid tumours, including breast, colon, lung, cervical cancer, and melanoma.11 For breast cancer, recent studies have reported that TIL may play an important role in determining prognosis and response to therapy. Retrospective analyses of tissue samples from multiple clinical trials have shown a strong relationship between high levels of TIL by immunohistochemistry and improved outcomes.12 The HER2+ and TNBC subtypes appear to have the highest TIL expression13 and across these two subtypes there is a correlation between the presence of TIL and increased levels of programmed cell death ligand-1 (PD-L1) expression.14 Some hormone receptor+ breast cancers also demonstrate high TIL levels. Thus, strategies that augment innate antitumour immune activity, or induce immune activity in immunologically ‘cold’ tumours, may be relevant across breast cancer subtypes. In this review, the authors summarise the biologic rationale for immune modulation in breast cancer, describe and discuss breast cancer immunotherapy clinical trial data presented or published to date, and highlight some ongoing clinical trials that are potentially poised to change future oncology practice, with a focus on immune checkpoint inhibitors (ICI).
THE BIOLOGY BEHIND IMMUNOTHERAPY IN BREAST CANCER
The first evidence implicating the immune system in controlling tumour growth came from Dr William Coley, who administered bacterial fragments (which became known as ‘Coley’s toxins’) to induce tumour shrinkage in the 1890s.15 Numerous clinical trials have since tested vaccines developed from inactivated cancer cells with very few clinical responses.16 Great progress was made after scientists focussed their research on cancer immunology to map the molecular mechanisms of T-cell antigen recognition, regulation, and function. A crucial step was understanding how the immune system balances an active immune phenotype to prevent autoimmunity, and the subsequent discovery of immune checkpoint molecules.17 These molecules work as negative co-stimulatory signals to attenuate T-cell responses to foreign antigens.18 Normal immune system response to nonself-antigen requires a step-wise process: a T cell recognises and binds to an antigen presented by antigen presenting cells (APC; Signal 1), then a B7 ligand binds to a T-cell co-stimulatory molecule (Signal 2) to enhance T-cell activation and proliferation.19 If this process is interrupted, the immune system interprets the antigen as ‘self’, leading to tolerance.20 On the other hand, if Signal 2 is uncontrolled, it may lead to normal tissue damage and severe host injury.18 Checkpoint molecules allow the immune system to achieve homeostasis, but cancer cells can use this interaction to prevent an anticancer immune response.21
First identified in 1987, cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) is a member of the Ig superfamily and homologous to the T-cell co-stimulatory protein CD28. It was the first molecule identified as a checkpoint co-inhibitory molecule.17 CTLA-4 becomes upregulated on the T cell surface, ultimately binding B7 in competition with CD28, leading to suppression of T cells and functioning as a co-inhibitory signal.22 Blocking the CTLA-4 receptor with therapeutic antibodies unleashes an immune response, as demonstrated in mouse model studies23 (Figure 1). This discovery led to a radical shift in cancer immunotherapy, moving from the initial concept of activating the immune system to attack cancer cells via vaccination, to a strategy of removing the co-inhibitory signal that blocks T cell responses.
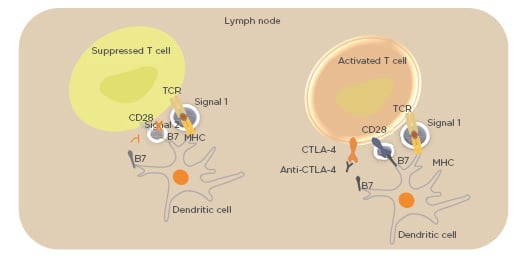
Figure 1: Binding of CTLA-4 to B7 costimulatory molecule blocks immunological Signal 2, impeding T-cell activation.
Using monoclonal antibodies to block CTLA-4 releases signal by CD28, enabling Signal 2 and T-cell activation.
CD28: cluster of differentiation 28; CTLA-4: cytotoxic T-lymphocyte antigen-4; MHC: major histocompatibility complex; TCR: T-cell receptor.
In the early 2000s, scientists identified the programmed cell death-1 (PD-1) inhibitory receptor and its ligands (PD-L1 and PD-L2) as additional members of the co-inhibitory pathway maintaining T-cell tolerance and prevention of autoimmunity.24 PD-1 is expressed on T cells, B cells, natural killer cells, monocytes, and dendritic cells, but its functional and biochemical properties have been best studied in T cells.25
Upregulation of PD-1 ligands in the tumour microenvironment and their ligation to PD-1 on CD8+ T cells is a key mechanism by which cancer cells limit the host immune response.26 PD-1 is expressed on T cells after they have been activated. The PD-1/PD-L1 inhibitory mechanism leads to a selective suppression and exhaustion of tumour-specific T cells. Thus, therapeutic targeting of the PD-1/PD-L1 inhibitory mechanism may impede T-cell exhaustion, and thus reinvigorate tumour-specific T cells to destroy the cancer27 (Figure 2).
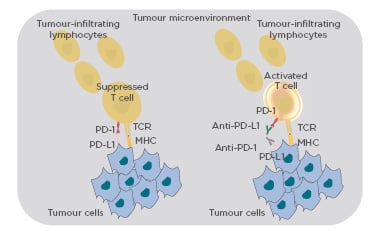
Figure 2: Binding of PD-1 to PD-L1 suppresses the effect of the T-cell receptor.
Using monoclonal antibodies to block PD-1 or PD-L1 releases the suppression, activating the T cell.
MHC: major histocompatibility complex; PD1: programmed cell death-1; PD-L1: programmed cell death ligand-1; TCR: T-cell receptor.
IMMUNE CHECKPOINT INHIBITORS IN BREAST CANCER: CURRENT CLINICAL EXPERIENCE
Most immunotherapy clinical trials in breast cancer have focussed on CTLA-4 and PD-1/PD-L1 inhibition. Two monoclonal antibodies that inhibit CTLA-4 have been studied in breast cancer: tremelimumab, a fully humanised IgG2 monoclonal antibody, and ipilimumab, a fully humanised monoclonal IgG1 antibody specific to human CTLA-4. Tremelimumab has no U.S. Food and Drug Administration (FDA)-approved indication. Ipilimumab is FDA-approved, both as monotherapy for melanoma and in combination with the anti-PD-1 inhibitor nivolumab for the treatment of melanoma,28 non-small cell lung cancer,29 renal cell cancer, and some colorectal cancers. There are five monoclonal antibodies targeting the PD-1/PD-L1 pathway with published clinical trials in breast cancer: the anti-PD-1 monoclonal antibodies pembrolizumab and nivolumab, and the anti-PD-L1 monoclonal antibodies atezolizumab, durvalumab, and avelumab. Table 1 highlights the completed and ongoing clinical trials discussed in this paper.
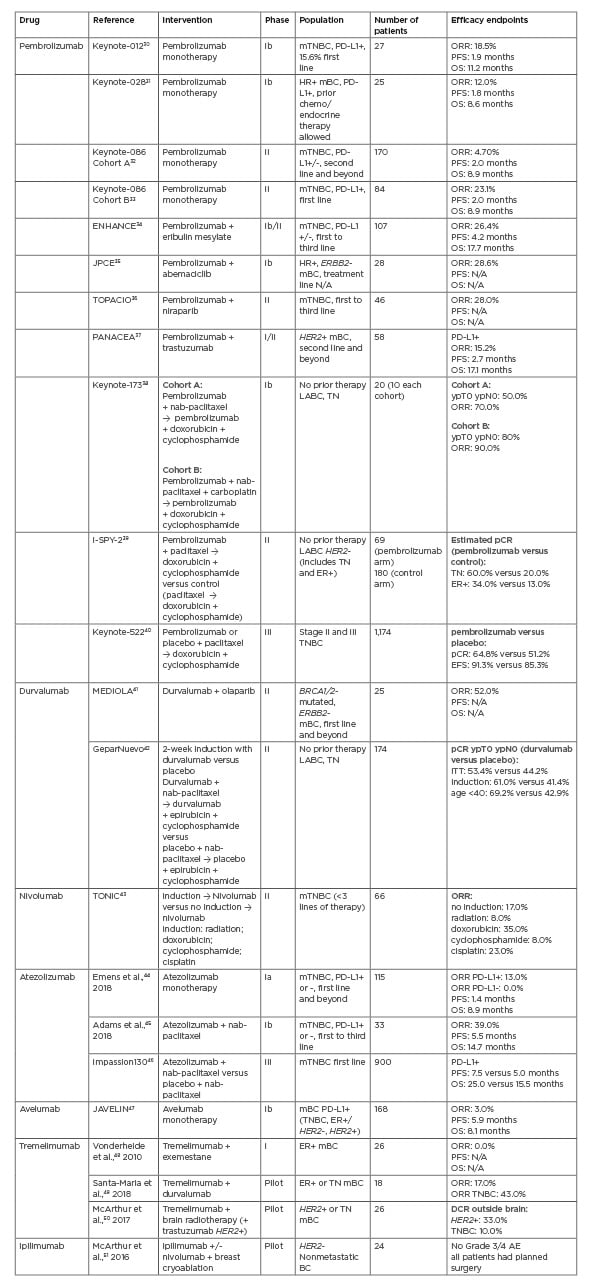
Table 1: Reported clinical trials with immune checkpoint inhibitors in metastatic or early breast cancer.
AE: adverse events; BC: breast cancer; DCR: disease control rate; ER: oestrogen receptor; ERBB2: Erb-B2 receptor tyrosine kinase 2; HER2: human epidermal receptor 2; ITT: intention to treat; LABC: locally advanced breast cancer; mBC: metastatic breast cancer; mTNBC: metastatic triple-negative breast cancer; ORR: overall response rate; OS: overall survival; pCR: pathologic complete response; PD1: programmed cell death-1; PD-L1: programmed cell death ligand-1; PFS: progression-free survival; TN: triple negative; TNBC: triple-negative breast cancer.
CTLA-4 INHIBITORS IN BREAST CANCER
Tremelimumab
In a small Phase I trial of tremelimumab in combination with exemestane, no objective responses were observed in a heavily pretreated ER+ MBC population.48 Stable disease after 12 weeks occurred in 42% of patients, with one-third having previously progressed on exemestane. The most frequent Grade 1 or 2 treatment-related adverse events (TRAE) were diarrhoea (46%), pruritus (42%), constipation (23%), and fatigue (23%). Five patients experienced Grade 3 TRAE. There were no Grade 4 TRAE.48 Only one serious AE was observed, related to diarrhoea, pyrexia, and dehydration, not responding to oral steroids, and requiring hospitalisation. In a single-arm, open-label pilot study of tremelimumab and durvalumab, an overall response rate (ORR) of 17% was observed in 18 evaluable patients with treatment-resistant metastatic HER2-negative (-) breast cancer (0% and 43% ORR in the ER+ and TNBC cohorts, respectively).49 Hepatitis, rash, and electrolyte abnormalities were the most common TRAE. No Grade 4 or 5 TRAE were observed. Several additional clinical trials exploring combination systemic strategies with tremelimumab in breast cancer are ongoing.52-54
Local strategies, such as cryoablation and radiation, invoke inflammation and may improve antigen presentation by destroying tumour integrity.
Numerous preclinical studies have demonstrated that cryoablation and radiation synergise with ICI to prevent tumour rechallenge.55 Consequently, there is considerable interest in clinical strategies that combine local therapies with ICI. In one such study of tremelimumab with brain radiation for the treatment of breast cancer brain metastases, the disease control rate in the nonirradiated, non-central nervous system metastases was 33% and 10% in the HER2+ and TNBC cohorts, respectively.50 An expansion of the study’s HER2+ cohort is planned. A trial exploring ICI with hypofractioned radiation therapy for breast cancer and other tumours is underway.56 A further discussion of the role of cryoablation and immunotherapy in breast cancer is outlined below.
Ipilimumab
A pilot study to investigate the safety and feasibility of ipilimumab in combination with tumour cryoablation enrolled 19 patients with HER2- early-stage breast cancer, for whom mastectomy was planned, to receive cryoablation alone (Group A), single-dose ipilimumab at 10 mg/kg (Group B), or both cryoablation and single-dose ipilimumab at 10 mg/kg (Group C).51 The results showed that cryoablation and ipilimumab was safe and well-tolerated, alone and in combination. Importantly, there was no surgery delay and no Grade 3 or 4 TRAE.51 The combination increased Th1-cytokine production, peripheral T-cell proliferation and activation, and intratumoural proliferation of effector T cells relative to regulatory T cells indicating both local and systemic antitumour immunity.57 After a median 66 months of follow-up, no recurrences have been reported as yet, and an expansion with five patients treated with ipilimumab and nivolumab demonstrated no new safety signal.58 This strategy is now being explored in a randomised Phase II study of cryoablation with ipilimumab and nivolumab versus standard of care in women with residual TNBC after standard-of-care chemotherapy for early-stage disease, a high-risk population with a 40% risk of recurrence.52 Other Phase II clinical trials are actively recruiting patients with breast cancer for treatment with ipilimumab in combination with other therapies.53,54
PD1/PD-L1 INHIBITORS IN BREAST CANCER
Pembrolizumab
Pembrolizumab is a highly selective humanised monoclonal antibody against PD-1 with clinical data in patients with TN, ER+, and HER2+ breast cancer. One of the first trials of pembrolizumab for breast cancer was the Keynote-012 trial, wherein patients with chemotherapy-resistant metastatic PD-L1+ TNBC were treated with single agent pembrolizumab.30 Safety was the primary endpoint with 56.3% of patients experiencing at least one TRAE, including 15.6% that were Grade 3–5. In the 27 patients who met the criteria for efficacy analysis, the ORR was 18.5%.30 In Cohort A of the Keynote-086 study, patients with chemotherapy-refractory metastatic TNBC were enrolled and pembrolizumab was associated with an ORR of 5.3% and 5.7% for the total cohort and PD-L1+ subset, respectively.32 In Cohort B of Keynote-086, 84 patients with PD-L1+ TNBC received single-agent pembrolizumab as first-line palliative therapy and an ORR of 21.4% was observed, indicating improved efficacy with administration earlier in the course of disease.33 In the Phase I/II ENHANCE-1 trial, pembrolizumab combined with eribulin mesylate conferred an ORR of 26.4% in patients who had received 0–2 prior lines of therapy for metastatic TNBC, regardless of PD-L1 status.34 In the Phase II TOPACIO trial, pembrolizumab was administered with niraparib, a PARP inhibitor, in 55 patients with metastatic TNBC, with a confirmed ORR of 21% reported.36
The Keynote-028 trial evaluated efficacy and safety of pembrolizumab monotherapy in patients with metastatic, PD-L1+, hormone therapy, and chemotherapy-resistant ER+ breast cancer, with an ORR of 12% reported. Among the 25 evaluable patients, 64% experienced at least one TRAE and 16% experienced Grade 3 or 4 AE.56 Pembrolizumab has also been tested with the cyclin-dependent kinase 4/6 inhibitor abemaciclib in a Phase Ib study with ER+, HER2-, previously treated metastatic breast cancer.49 For the 28 evaluable patients, the ORR was 14.3%, with no concerning safety signals identified. The Phase Ib/II PANACEA trial demonstrated activity with pembrolizumab and trastuzumab in a trastuzumab-resistant HER2+ metastatic breast cancer population.37 In the second phase of this trial, a 15% ORR and a 25% disease control rate were observed in the PD-L1+ participants.
In the curative-intent setting, combination strategies with pembrolizumab and chemotherapy have been tested in multiple trials. Early data from the I-SPY2 trial revealed that adding pembrolizumab to neoadjuvant paclitaxel prior to administration of neoadjuvant doxorubicin with cyclophosphamide resulted in an estimated pathologic complete response (pCR) rate of 34% versus 13% in hormone receptor+, HER2– patients, and 60% versus 20% in TNBC patients.39 The Keynote 173 study is a multicentre six-arm Phase Ib trial of pembrolizumab plus chemotherapy with TNBC patients suited to neoadjuvant therapy.38 Initial results comparing Arm A (without carboplatin) versus Arm B (with carboplatin) revealed pCR in breast and axilla of 50% and 80%, respectively.38 In this small trial, all patients presented with TRAE, but all were nonfatal and few patients discontinued study treatment due to AE. The first Phase III data that confirmed the benefit of adding pembrolizumab to neoadjuvant chemotherapy in TNBC came from the Keynote 522 trial.40 This international multicentre trial randomised 1,174 patients in a 2:1 fashion to pembrolizumab plus chemotherapy versus placebo plus chemotherapy for 24 weeks, followed by surgery and adjuvant pembrolizumab or placebo for 27 weeks. Chemotherapy consisted of carboplatin plus neoadjuvant paclitaxel and doxorubicin plus cyclophosphamide. Primary endpoints were pCR and event-free survival. In the first interim analysis with a median 18-month follow-up, pCR rates were significantly increased in the pembrolizumab arm compared to placebo (64.8% versus 51.2%; p=0.00055). This benefit was independent of the PD-L1 status, although almost 80% of patients were PD-L1+. There was a strong favourable trend for an event-free survival benefit with the addition of pembrolizumab, but it has not yet met the predefined boundaries of statistical significance.40 Toxicity was mostly related to the chemotherapy backbone. The most common immune-mediated AE were infusion reactions (17.7%) and hypothyroidism (14.9%). One patient in the pembrolizumab arm died from pneumonitis. Several randomised Phase III clinical trials with pembrolizumab in different breast cancer subtypes and clinical settings are underway.59-63
Nivolumab
Nivolumab is an anti-PD-1 antibody with confirmed efficacy and safety in several tumour types, including melanoma, lung cancer, urothelial carcinoma, renal cell carcinoma, hepatocellular carcinoma, microsatellite instability-high colorectal cancer, head and neck cancer, and Hodgkin’s lymphoma.64 In breast cancer, the TONIC trial investigated five strategies for induction therapy in metastatic TNBC including radiation therapy, doxorubicin, cyclophosphamide, cisplatin, or no induction treatment followed by nivolumab therapy.43 The ORR was highest in the doxorubicin group at 35%, followed by 23% in the cisplatin arm, 17% in the no induction followed by nivolumab arm, and 8% in both the radiation and cyclophosphamide arms. In the nivolumab treatment phase, 20% of patients experienced Grade 3–5 TRAE.43 To further explore clinical activity of nivolumab in breast cancer, randomised Phase II clinical trials are ongoing.52-54
Atezolizumab
Atezolizumab is a humanised IgG1 monoclonal antibody that targets PD-L1. In a Phase Ia trial, atezolizumab was studied in 115 patients with metastatic TNBC. The unconfirmed ORR was 13% in PD-L1+ tumours and 5% in PD-L1- tumours.44 Combination therapy with atezolizumab and chemotherapy was first tested in a Phase 1b trial that enrolled patients with metastatic TNBC, regardless of the PD-L1 status.45 Although safety was the primary endpoint, the ORR was 39.5%. Responses occurred in patients with both PD-L1+ and PD-L1- disease and were higher in first versus later-line settings with an ORR of 53.8% and 30.0%, respectively. Grade 3–4 haematologic toxicity occurred in more than half of the patients, but this was manageable.45 Based on these findings, a Phase III randomised clinical trial was designed and recently published.46 Over 900 patients with previously untreated metastatic TNBC were randomised to neoadjuvant paclitaxel/atezolizumab or neoadjuvant paclitaxel/placebo. With co-primary endpoints of progression-free survival and overall survival (OS) in the overall and PD-L1+ population, this trial gave overall positive results. There was a significant increase in progression-free survival in the overall and the PD-L1+ population. Although the OS benefit was not statistically significant in the overall population, there was a clinically significant increase in the median OS with the addition of atezolizumab in the PD-L1+ population (25.0 versus 15.5 months).46 The combination of atezolizumab plus neoadjuvant paclitaxel was recently approved in several countries for use in patients with metastatic TNBC. As a result, several randomised Phase III trials are being conducted in patients with breast cancer either in the metastatic setting or in the curative-intent scenario.65-69
Durvalumab
Durvalumab is another human monoclonal antibody that inhibits interaction between PD-1 and PD-L1. The drug has demonstrated safety and clinical activity in other tumour types, such as urothelial carcinoma, head and neck cancer, and non-small cell lung cancer.70-72 In the placebo-controlled GeparNuevo study, 174 patients with early-stage TNBC were randomised to standard neoadjuvant anthracycline/taxane chemotherapy with or without durvalumab.42 In the window-phase, 117 patients received durvalumab or placebo 2 weeks prior to the start of chemotherapy. Treatment with durvalumab resulted in a higher pCR rate, 53.4% versus 44.2%, but this result did not reach the predefined statistical endpoint. In the predefined subgroup analysis, patients receiving durvalumab in the window-phase had significantly higher pCR rates compared to placebo (61.0% versus 41.4%; p=0.052). The most common immune-related AE were thyroid dysfunction of any grade, which was experienced in 47.0% of patients.42 In the Phase II MEDIOLA trial, durvalumab was combined with olaparib, another PARP inhibitor, in 25 patients with BRCA1/2-mutated, HER2– metastatic breast cancer with a confirmed ORR of 52% reported.41 To further explore this drug efficacy and safety, several Phase II and III randomised clinical trials in breast cancer are open to accrual.73-77
Avelumab
Avelumab is another monoclonal antibody directed against PD-L1 with clinical activity and safety tested in several tumour types.78-79 JAVELIN Solid Tumour is an international, open-label, Phase I trial in patients with advanced solid malignancies being treated with avelumab. The Phase Ib breast cancer cohort included 168 previously treated patients.47 The ORR was 3.0% in the overall patient population. The ORR in the TNBC, HER2+, and hormone+ subtypes were 5.2%, 3.8%, and 2.8%, respectively.47 TRAE occurred in 68.0% of the patients with 13.7% experiencing Grade 3 or higher.47 Although limited clinical data are available with avelumab in breast cancer, several randomised clinical trials are under way in different clinical settings.80-84
CONCLUSION
Immunotherapy in breast cancer has proven its potential over years of treatments. From the preclinical rationale to registration Phase III trials, a great deal has been learnt about the efficacy and mechanisms of action. Specifically, ICI alone is safe, but has limited efficacy in the metastatic setting across subtypes based on early-phase trials.32,44,79 Strategies that increase antigen release and potentiate the ICI therapeutic index are being explored in numerous trials combining anti-PD-1/L1 with chemotherapy, targeted therapies, or locoregional therapies with the goal of increasing response rates and duration of response.34,45 In metastatic TNBC, first-line treatment with the anti-PD-L1 inhibitor atezolizumab and neoadjuvant paclitaxel chemotherapy is now the standard of care for patients with PD-L1+ tumours.46 Small Phase II trials of combination chemotherapy and anti-PD-1 monoclonal antibodies demonstrated a significant increase in pCR rates, but with added toxicity.38,39,42 Several randomised Phase III trials are under way, either in the curative intent or in the metastatic setting and exploring the combination of ICI and chemotherapy in treating ER+, HER2+, or TNBC. The future of immunotherapy, in particular ICI, for the treatment of breast cancer is very promising, and it is hoped that rationale ICI combination strategies will ultimately improve cure rates and advance the treatment landscape.







