Abstract
A 58-year-old female presented with a right axillary mass, which was confirmed as Stage IB BRAF-mutant melanoma based on the histology of the resected mass. The patient’s history included a left upper arm melanoma that was resected in 2012; an allograft renal transplant secondary to polycystic kidney disease from a deceased donor, which they had undergone in 2009; and immunosuppressive therapy, which they had been on since the transplant took place.
The patient relapsed 8 months after axillary clearance. Dual immunotherapy is the first-line treatment for BRAF-mutant melanoma, but it has been associated with high rates of solid organ graft rejection in systematic reviews. For this reason, targeted therapy with dabrafenib and trametinib was commenced in the first instance, which halted disease progression for 10 months. On progression, dual immunotherapy was again discussed, and the patient fully consented regarding risks of graft loss. They had an excellent treatment response, and their renal graft remains functional.
![]()
Erratum: Dual Immunotherapy in a Patient with Rapidly Progressive Metastatic Melanoma Without Failure of Grafted Kidney
Authors: Charles J Limula, Abigail Gee, Jonathan Potts, Hannah Taylor, Christopher Herbert, Helen Winter
Original citation: EMJ Oncol. 2022; DOI/10.33590/emjoncol/22-00002. https://doi.org/10.33590/emjoncol/22-00002.
Date correction published: 09.11.2022
In the article by Limula et al., published in EMJ Oncology on 07.05.22, Figure 2 was incorrect as image A was not included with image B.
In the same article Figure 4 was mislabelled as the figure title was originally as follows: “Figure 3: The estimated glomerular filtration rate over time as a guide for the renal function of a patient who had received a renal graft.” The title should have read: “Figure 4: Anteroposterior abdominal CT scans showing the renal graft (indicated by the arrow) before (A) and after (B) dual immunotherapy.”
The above have now been corrected.
EMJ apologises for the error and any inconvenience caused.
![]()
Key Points
1. This case review discusses a 58-year-old female diagnosed with a relapse BRAF-mutant melanoma on the left upper arm, and a history of renal transplant secondary to polycystic kidney disease. They were treated with dual immunotherapy (IO) and showed excellent treatment response.2. Dual IO has a better 5-year survival rate than using a single agent in the management of melanoma; however its use is challenging in patients with a history of organ transplant, as high rates of acute graft rejection have been shown in systematic reviews.
3. The authors concluded that patients with a kidney graft from a deceased donor can be administered dual IO safely, and can achieve good cancer response and graft tolerance. To aid the selection and monitoring of patients with a renal graft, predictive biomarkers for graft rejection following IO would be beneficial.
BACKGROUND
Melanoma is the third most common skin cancer in the UK.1 Checkpoint inhibitors (CPI) have revolutionised the treatment of melanoma.2-4 Dual immunotherapy (IO) with anti-cytotoxic T lymphocyte antigen 4 and anti-programme cell death protein 1 agents is associated with improved median progression-free survival.5,6 Clinical trials with CPIs have typically excluded patients with solid organ transplant due to concerns of organ rejection and loss.7-9 Several cases of acute graft rejection after the use of CPIs have been reported.10 This paper details the case of a patient with a renal transplant secondary to polycystic kidney disease and a diagnosis of relapsed BRAF-mutant melanoma, for whom dual IO was used and good treatment response and renal graft preservation were achieved.
CASE PRESENTATION
A 58-year-old female with a medical history of polycystic kidney disease had undergone an allograft kidney transplant from a deceased donor in 2009, and had since remained on an immunosuppressive regime of prednisolone (10 mg, once daily) and ciclosporin (150 mg, twice daily [BD]). The patient then had a left upper arm melanoma confirmed 2 years after the transplant, for which they underwent a complete resection with no residual malignancy. In 2018, the patient detected a lump in their right axilla, but was otherwise well. On examination, the patient was stable, but had a palpable right axillary mass without regional lymphadenopathy. An ultrasound-guided biopsy of the lump confirmed melanoma Stage IB (T2aN0M0). No metastases were found in the staging CT scan.
The patient underwent an axillary clearance; no adjuvant therapy was available at the time. The patient then developed pain 8 months later, and a further right axillary lump was noticed. A restaging CT scan confirmed metastases to the liver and lungs. Biopsy of the left axillary lymph node confirmed BRAF-mutant melanoma (Figure 1). The treatment options available were dual IO, the standard first-line treatment for BRAF-mutant metastatic melanoma, or alternatively targeted therapy with dabrafenib and trametinib. Due to the patient’s renal graft and the evidence available regarding graft-rejection risk, the patient was started on targeted therapy as this was thought to have a lower risk of graft loss.11
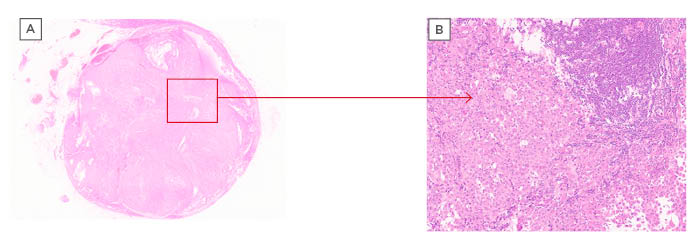
Figure 1: Microscope images of a left axillary lymph node at x10 magnification (A) and x100 magnification (B).
The box indicates the invasion of the node by BRAF-mutant metastatic melanoma, which can be seen in (B).
After five cycles of the targeted therapy, a restaging CT scan demonstrated a partial response. However, the patient became acutely unwell with severe back and abdominal pain after 11 months of targeted therapy. CT imaging confirmed extensive disease progression, this time including brain metastases. The patient required admission for symptom control of the rapidly progressive disease. They then gave consent for in-patient dual IO, clearly stating the high risk of graft loss. As a result of the strong evidence of graft loss with the use of IO in systematic reviews, it was anticipated that the patient would likely lose the grafted kidney.12-15 Single agent IO was considered and discussed with the patient, however, in view of extensive burden of the BRAF-mutant disease and expected poor prognosis, the patient made an informed decision to undergo dual IO with ipilimumab and nivolumab.
The patient made an informed decision to consent to treatment despite the risks of graft loss and the potential requirement for dialysis, which were highlighted to them. Due to their declining performance status, therapy was commenced as an inpatient procedure with close monitoring of renal function. After discussion with the renal team, the dose of ciclosporin was reduced from 150 mg BD to 50 mg BD, while the dose of prednisolone continued at 10 mg per day. The patient received all four cycles of dual IO (nivolumab plus ipilimumab) and further maintenance doses of nivolumab. The patient also received gamma knife therapy to the metastatic brain disease. They clinically improved after spending some time on the ward and were later discharged. The patient’s renal function was closely monitored and remained within acceptable limits (Figure 3).
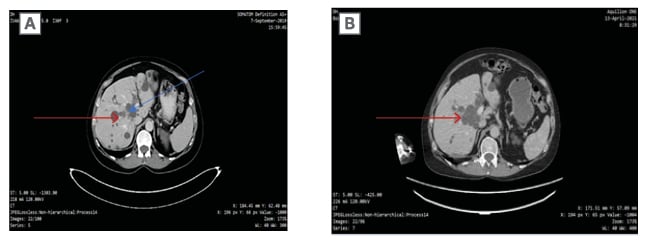
Figure 2: Abdominal CT imaging confirming extensive disease post-dual immunotherapy, with visible but not active lesions (indicated by the arrows).
FOLLOW-UP
CT staging at the end of four cycles confirmed a complete radiological response. The CT scan taken in April 2021 (Figure 2) confirmed a continued response with inactive lesions and graft preservation (Figure 4), and there were no active brain lesions. At the time of writing, the patient was clinically well, and although they tired more easily than before treatment, their pain was improved, they did not require regular analgesia, and they had a Eastern Cooperative Oncology Group (ECOG) performance status of 2.
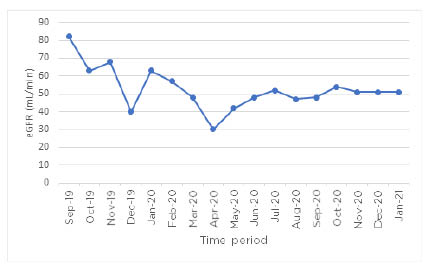
Figure 3: The estimated glomerular filtration rate over time as a guide for the renal function of a patient who had received a renal graft.
The patient was receiving dual immunotherapy for rapidly progressive BRAF-mutant metastatic melanoma. The graph shows an initial decline after commencing dual immunotherapy, but plateaus around 50 mL/min thereafter.
eGFR: estimated glomerular filtration rate.
CONCLUSION
Dual IO in the management of melanoma has been shown to have a better 5-year survival rate than when using a single agent.7,16-18 The use of dual IO in patients who have had a solid organ transplant remains challenging: systematic reviews have shown high rates of acute graft rejection of up to 81% across all solid organ grafts.12 In renal grafts alone, rates of up to 48% acute graft rejection have been reported, with a median rejection time of 21 days; therefore, the use of dual CPIs in patients who have had a transplant has been limited.12,19,20
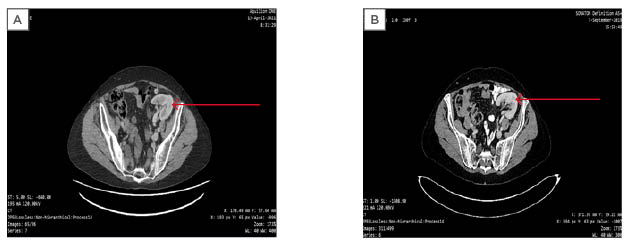
Figure 4: Anteroposterior abdominal CT scans showing the renal graft (indicated by the arrow) before (A) and after (B) dual immunotherapy.
This paper outlines the case of a patient with rapidly progressive melanoma for whom dual IO was safely delivered, resulting in complete response. The patient’s kidney function remained within acceptable limits, and their renal graft was preserved. The importance of enabling the patient to make informed treatment choices, and of balancing the benefits of therapy with the risks of predictable side effects, is key.21 Dose modification of immunosuppressive therapy has been demonstrated to be safe, without leading to graft rejection or loss of IO efficacy.
This case demonstrated that dual IO can be safely administered in patients with a kidney graft from a deceased donor, and can achieve good cancer response and graft tolerance. However, predictive biomarkers for graft rejection following IO would aid the selection and monitoring of patients with a renal graft. This case indicated that CPIs can be considered in patients with solid organ grafts and can achieve graft preservation. Patients should receive counselling regarding the potential of graft loss, and close monitoring of renal function is recommended. Dual IO remains the first-line treatment for patients with BRAF-mutant metastatic melanoma. For patients who have received a renal transplant, the optimal sequencing of treatment is yet to be determined.








