Abstract
Breast cancer is one of the deadliest cancer types worldwide and consists of several subtypes differing in their molecular characteristics; each subtype requires various effective treatment strategies. Development of resistance to radiation or therapeutic agents is one of the main factors leading to the death of about 450,000 breast cancer patients each year. Since microRNAs (miRNAs) have been shown to be key players in health and disease, it is not surprising that they influence the development of resistance to treatment and thereby affect the fate of patients suffering from different types of cancer. miRNAs typically modulate the expression of hundreds of targets, forming a complex regulatory layer which we have only begun to understand. This review summarises miRNAs that confer resistance to different treatment options or sensitise breast cancer cells to a particular treatment. Moreover, this review addresses the high clinical value of miRNAs as biomarkers that allow prediction or monitoring therapy response. The focus of the review is to illustrate how much we know already but also to emphasise that a vast part of the miRNome and its implications for breast cancer therapy resistance remains in the dark and requires further investigation.
BACKGROUND
Breast cancer is one of the most prevalent cancer types worldwide with approximately 1.3 million cases and 450,000 deaths each year.1 In spite of its apparent clinical and biological heterogeneity, it can be grouped into six clinical subtypes based on gene expression profiling of a 50-gene signature (PAM50) or immunohistochemical markers.2 The subtypes include: Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER2)-enriched, claudin-low, basal-like, and normal breast-like.3,4 The Luminal subtypes are characterised by expression of the oestrogen receptor (ER) and can thus potentially be targeted by endocrine therapy.3 Here, the Luminal A subtype has a far better prognosis and reduced relapse rate, mostly due to its higher ER expression, compared with the Luminal B subtype which is characterised by higher Ki67 staining and often additional overexpression of HER2, also named ERBB2.3,5 The HER2-enriched subtype is defined by amplification in the locus of the HER2 gene. Though it has been known to be quite aggressive and metastasis-prone, patients suffering from HER2-positive (HER2+) breast cancer largely benefited from the clinical success of the trastuzumab monoclonal antibody which targets the HER2 receptor overexpressed or amplified in this subtype.3 Basal-like breast cancer is known for its poor prognosis and limited therapy options.6 It largely resembles the immunohistochemically defined subtype of triple-negative breast cancer (TNBC). The two subtypes, claudin-low and normal breast-like, are currently rather poorly characterised and due to the lack of targeted therapies, their treatment is mainly limited to chemotherapeutics and radiation, similar to the basal-like subtype.3,7
The occurrence of resistance to the targeted treatments of these subtypes has been well documented. As endocrine treatment is used for the ER-positive (ER+) patients, the resistance to this approach is mainly mediated by the downregulation of ER or its corresponding signalling pathway. Similarly, as trastuzumab treatment in HER2-enriched patients depends on expression of the receptor, the resistance is facilitated by downregulation of HER2, upregulation of other members of the ErbB-family, or mutations in downstream signalling molecules leading to constitutive activation of survival and proliferation pathways.8 In contrast, resistance to conventional chemotherapy is often mediated by mechanisms that affect the metabolism of the drugs or their concentration within the cells, e.g. by the upregulation of efflux pumps. General resistance mechanisms usually involve desensitisation of cells to apoptosis or cell cycle arrest in response to genotoxic stress or pathway inhibition.
The primary microRNA (miRNA) is the product transcribed from the miRNA locus and is commonly produced by Pol ll.9 It is characterised by a hairpin structure and gets further processed by the RNAse Drosha, creating the precursor miRNA, which is then exported from the nucleus via Exportin 5.10 In the cytoplasm, Dicer further cleaves the transcript, creating the mature miRNAs of 21–25 nt in length with 5’ phosphate groups and a 3’ overhang of two nucleotides. Mature miRNAs then associate with Argonaute proteins, forming the RNA-induced silencing complex.11 In recent years, it has been shown that the miRNA processing machinery does not only produce two miRNA species from one precursor, namely the miRNA-3p and 5p, but can also give rise to so-called isomiRs. These are shifted forms of the canonical miRNAs derived from alternative processing displaying either altered stability (3’isomiRs) or altered seed sequences (5’isomiRs).12 A miRNA usually exerts its function on its target RNAs by inducing Argonaute-dependent degradation or translational repression and subsequent Argonaute-independent degradation via perfect or mismatch complementarity, respectively.9 For target recognition, only the so-called seed sequence, comprising nucleotides 2–8 of the miRNA, is essential.13 While the miRNA usually binds in the 3’ untranslated region of its target RNA, there are reports showing that it is also able to bind the 5’ untranslated region or open reading frame and induce translational activation.9
This review article will summarise current literature on the impact of miRNAs on therapy resistance in breast cancer by dissecting the role of individual miRNAs and their identified targets in the underlying cellular processes. The gathered information is based exclusively on data obtained from cell culture or mouse experiments. Moreover, the review highlights the main miRNAs involved in therapy resistance by impairing pluripotency, cancer stem cell properties, or epithelial–mesenchymal transition (EMT) of breast cancer cells. Furthermore, it will also demonstrate the pleiotropic nature of miRNAs using miR-200c as an example which has been shown to affect at the same time the cellular response to conventional chemotherapy, radiotherapy, and targeted treatment of HER2 or ER.
MECHANISMS OF microRNAS IN CHEMOTHERAPY AND RADIOTHERAPY RESISTANCE
Several miRNAs have been shown to mediate resistance to various chemotherapeutic drugs or to radiotherapy by targeting general cellular mechanisms induced by the drugs, such as cell cycle arrest, apoptosis, and impairment of DNA repair. Their targets and modes of action are summarised in Figure 1. Among those miRNAs targeting components of the DNA repair machinery, which are implicated in chemotherapy resistance or sensitivity, are miR-28, miR-181, miR-182, and miR-146, all four targeting BRCA1. Others, such as miR-155, miR-96, miR-107, and miR-221/222, target RAD51, while miR-203 and miR-181 repress expression of ATM. Further targets include H2AX (miR-138), WEE1 (miR-15), TP53 (miR-125b), and BCL2 (miR-34a).14 Moreover, various miRNAs with a role in chemotherapy resistance have been shown to enhance tumour progression by activating signalling pathways important for proliferation, cell cycle progression, and survival of the cancer cell. In addition, alterations in the methylation or histone modification pattern of the DNA can also be caused by miRNAs and thereby contribute to resistance. Further general mechanisms exploited by oncogenic miRNAs may also affect the availability of the drug. Transporters and metabolic enzymes, for instance, often play a role in decreasing the abundance of the drug. However, all these mechanisms can also be targeted by tumour-suppressive miRNAs enhancing sensitivity to chemo/radiotherapy and thus increasing the effectiveness of the treatment.15,16
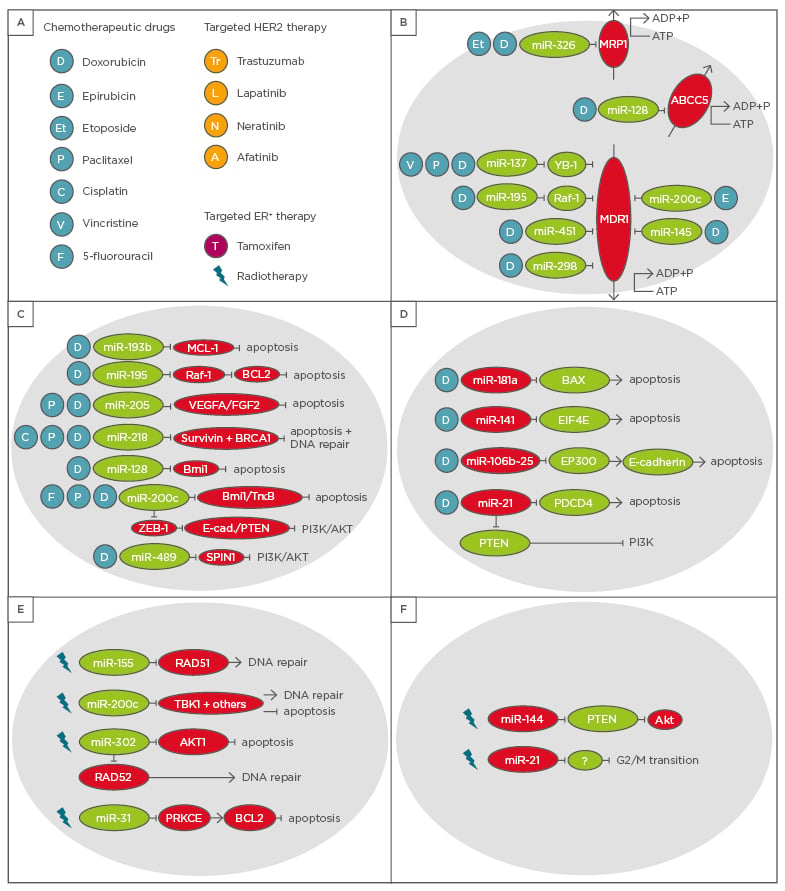
Figure 1: Overview of microRNAs with an impact on chemotherapy and radiotherapy in breast cancer patients.
A) These microRNAs can affect either B) efflux pumps, C) and D) apoptosis and DNA repair in response to chemotherapeutic agents, or E) and F) irradiation.
HER2: human epidermal growth factor receptor 2; ER+: oestrogen receptor positive.
microRNAs as Sensitisers to Chemotherapy
Chemotherapeutic drugs mainly target cell proliferation (anthracyclines) or induce severe DNA damage to cause cell cycle arrest or apoptosis (taxanes and platinum compounds).17
Combinations of anthracyclines, such as doxorubicin, daunomycin, and epirubicin; and taxanes, such as paclitaxel and docetaxel, are among the most common treatment strategies for advanced breast cancer.18 Among the most commonly used neoadjuvant regimens, which are administered before surgery or radiotherapy, are combinations of paclitaxel, docetaxel, doxorubicin, epirubicin, cyclophosphamide, and fluorouracil.19
One of the most efficient mechanisms by which cancer cells develop resistance towards chemotherapy is a high abundance of efflux transporters, which remove the chemotherapeutic drug from the cells. Multidrug resistance-associated protein 1 and multidrug resistance protein 1 (MDR1), for instance, belong to this group of transporters. Several miRNAs, including miR-451, miR-145, miR-298, miR-200c, and miR-326, have been shown to reduce expression of MDR1 and thereby sensitise breast cancer cells to anthracyclines.20-23 miR-326, however, also increases the vulnerability of breast cancer cells to etoposide treatment by targeting MDR1.24 Furthermore, high levels of miR-195 enhance the sensitivity of breast cancer cells to doxorubicin by reducing Raf-1 levels, which induces apoptosis via downregulation of BCL2 and also represses MDR1 levels.25 miR-137 was also shown to render breast cancer cells more susceptible to various chemotherapeutic drugs (doxorubicin, vincristine, and paclitaxel) by targeting YB-1, which suppresses MDR1 levels.26 Further miRNAs involved in chemotherapy resistance by affecting drug efflux via different direct targets are miR-7, miR-127, miR-134, miR-196a, miR-221/222, miR-508-5p, miR-129-5p, miR-103/107, miR-9, and miR-519c.27
Several other miRNAs are known to sensitise breast cancer cells to chemotherapeutic drugs by inducing apoptosis or preventing DNA damage repair which generally results in cell cycle arrest or apoptosis. One of these miRNAs is miR-193b which increases the sensitivity of breast cancer cells to doxorubicin treatment by directly targeting myeloid cell leukaemia-1, thereby inducing apoptosis.28 Another miRNA sensitising breast cancer cells to certain chemotherapeutic drugs is miR-489. In addition to VAV3, BCL2, and AKT3, SPIN1 was identified as a direct target of miR-489. Downregulation of SPIN1 or overexpression of miR-489 inhibited activation of the PI3K-Akt pathway and consequently increased sensitivity to doxorubicin. Blockage of the PI3K-Akt pathway enhanced cell death in the cancer cells.29 Furthermore, high abundance of miR-218 reversed the resistance of breast cancer cells to doxorubicin as well as paclitaxel by inducing apoptosis. Knock-down of survivin (BIRC5), a direct target of the miRNA, was able to phenocopy the increase in apoptotic cells after treatment with the respective drugs.30 Interestingly, this miRNA also targets BRCA1 to enhance the sensitivity of breast cancer cells to cisplatin treatment by inducing apoptosis and lowering their ability to repair DNA damage.31 Enhancement of chemosensitivity to doxorubicin and paclitaxel can also be mediated by miR-205 which directly targets VEGFA and FGF2 and thereby induces apoptosis.32
In the case of miR-200c, several additional mechanisms to the above-mentioned downregulation of MDR1 have been described to sensitise breast cancer cells to various drugs. For instance, direct targeting of the tyrosine kinase receptor TrkB and the transcriptional repressor Bmi133 or inhibition of Akt signalling via E-cadherin and PTEN, which are both upregulated upon ZEB1 suppression by miR-200c, sensitise cells to doxorubicin. Moreover, miR-200c overexpression causes an increase in the sensitivity of breast cancer cells to 5-fluorouracil treatment by lowering Bmi1 levels.34 Another miRNA targeting Bmi1 and thereby enhancing sensitivity of breast cancer cells to doxorubicin is miR-128. A second direct target of miR-128 is the transporter ABCC5. Overexpression of the miRNA decreases Bmi1 and ABCC5 levels, leading to a significant increase in the number of apoptotic cells and cells with DNA damage enhancing doxorubicin effectiveness.35
microRNAs Conferring Resistance to Chemotherapy
miR-181a induction upon treatment with DNA damaging compounds such as doxorubicin was linked to chemoresistance in breast cancer. Upregulation of the miRNA after treatment with doxorubicin required STAT3 activation via the NF-κB pathway. Decreased BAX levels, a direct target of miR-181a, help breast cancer cells to resist apoptosis.36 Overexpression of miR-141 rendered breast cancer cells resistant to the drug docetaxel. Direct repression of eukaryotic translation initiation factor 4E levels by the miRNA allowed the cells to circumvent drug-induced apoptosis.37 Breast cancer cells overexpressing the miR-106b~25 cluster acquired resistance to doxorubicin. miR-25 is especially important for developing resistance, however, all three miRNAs in the cluster target the transcriptional E-cadherin activator EP300 and other unidentified targets to reduce sensitivity to anti-cancer treatments.38
microRNAs with a Role in Radiotherapy Resistance
During radiotherapy, beams of ionising radiation are aimed at the tumour and damage the DNA of tumour cells. Resulting DNA double-strand breaks are the predominant lesions caused by radiotherapy.39 Therefore, the targets of miRNAs mediating radiotherapy resistance or enhancing sensitivity to radiotherapy are mainly connected to the DNA repair machinery or apoptosis. miR-155, for instance, lowers the efficiency of breast cancer cells repairing the radiation-induced DNA damage via homologous recombination by targeting RAD51,40 whereas overexpression of miR-200c promotes radiation-induced cell death in breast cancer cells and sensitises them to DNA damage upon radiation. TBK1 was identified as a direct target of miR-200c, but TBK1 knock-down could not account for the complete effect miR-200c has on the cellular response to radiotherapy.41 The function of miR-302 is also tumour-suppressive since this miRNA directly targets AKT1 and RAD52, both mediators of radioresistance. In vitro as well as in vivo experiments showed that miR-302 overexpression in TNBC cells reversed resistance to radiotherapy, made the cells even more sensitive to radiation, and suppressed AKT1 and RAD52 levels.42 In contrast, high levels of miR-144 enhanced radiotherapy resistance of breast cancer cell lines. A strong decrease in the PTEN levels, a direct target of miR-144, caused AKT activation.43
microRNAs and Their Role in Resistance to Chemotherapy and Radiotherapy
So far, few miRNAs have been shown to mediate or counteract resistance to chemotherapy as well as radiotherapy. One example is the tumour- suppressive miR-31, which inhibits the NF-κB pathway by directly targeting PRKCE and thereby inducing apoptosis and increasing sensitivity to chemo and radiotherapeutic treatment in MDA-MB-231 cells, a TNBC cell line. The downregulation of PRKCE leads to a decrease in BCL2 which accounts for the increase in sensitivity to doxorubicin treatment and radiation since the presence of BLC2 exerts an anti-apoptotic effect.44 miR-21 on the other hand, exerts an oncogenic function by mediating resistance to radiotherapy as well as chemotherapy. miR-21 prevents the breast cancer cells from entering G2/M arrest after radiation. However, the direct targets helping to overcome the G2/M arrest upon radiation have not been identified yet.45 In terms of chemotherapy resistance, miR-21 has been shown to confer resistance to doxorubicin by directly targeting the tumour-suppressor proteins PTEN46 and PDCD447 which prevents the cells from undergoing apoptosis and mediates the drug resistance.
SPECIFIC MECHANISMS OF microRNAS IN RESISTANCE TO TARGETED THERAPY
Mechanisms and targets of miRNAs impacting the sensitivity of breast cancer cells to targeted therapies are summarised in Figure 2.
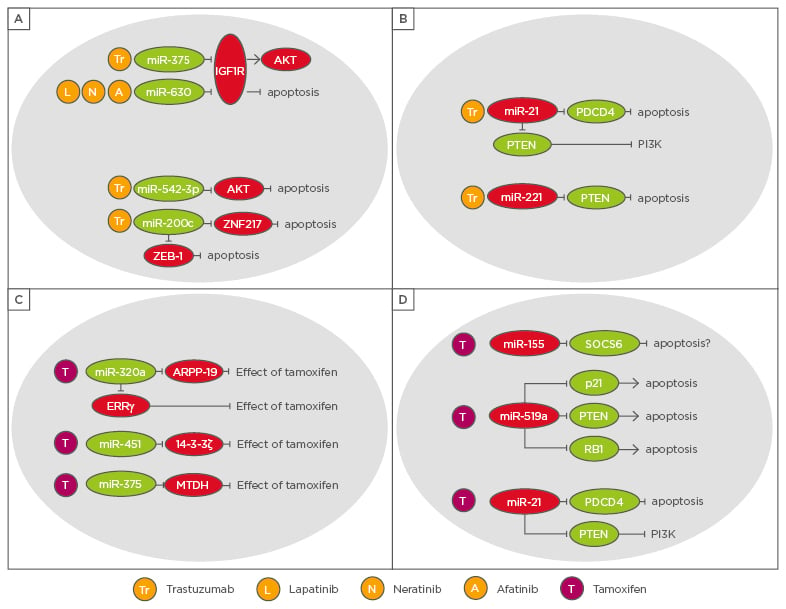
Figure 2: Overview of microRNAs with an impact on targeted therapies in breast cancer patients.
A) and B) microRNAs with a role in HER2 overexpressing patients, C) and D) microRNAs with a role in resistance to endocrine therapy.
ERRγ: oestrogen-related receptor gamma.
microRNAs in Resistance to Targeted Therapy for Human Epidermal Growth Factor Receptor 2 Patients
Targeted therapy approaches for HER2 patients use drugs antagonising or blocking the HER2 receptor. These include trastuzumab or pertuzumab, monoclonal antibodies targeting the HER2 receptor, and the tyrosine kinase inhibitors lapatinib, neratinib, and afatinib.48 The loss of miR-375 due to epigenetic silencing contributes to trastuzumab resistance. The underlying mechanism involves insulin-like growth factor type 1 receptor (IGF1R) as a direct target of the miRNA. In the absence of miR-375, IGF1R can function as alternative growth factor receptor in breast cancer patients treated with trastuzumab.49 Another miRNA targeting IGFR1 and thereby increasing the effectiveness of drugs targeting HER2, such as lapatinib, neratinib, and afatinib, is miR-630.50 miR-542-3p also enhances sensitivity to trastuzumab treatment however, via targeting AKT. The loss of mIR-542-3p renders the HER2+ breast cancer cells more resistant to the drug, reduces the number of apoptotic cells, and enables inhibition of the G1/S checkpoint to be overcome.51 The tumour-suppressive function of miR-200c not only sensitises breast cancer cells to chemotherapeutic drugs such as doxorubicin, 5-fluorouracil, and epirubicin,22,33,34,52 it also sensitises HER2+ breast cancers to trastuzumab via downregulation of ZNF217 and ZEB1.53 In terms of trastuzumab and lapatinib resistance, miR-16 has been shown to increase the sensitivity of breast cancer cells by targeting cyclin J and far upstream element-binding protein 1. Moreover, miR-16 might serve as a potential biomarker to predict the therapy response of HER2 patients.54 A miRNA with an oncogenic role in targeted therapy resistance of HER2 patients is miR-221, which prevents breast cancer cells from undergoing apoptosis and mediates trastuzumab resistance in HER2+ breast cancer cells by targeting PTEN.55 miR-21, another oncogenic miRNA, not only mediates resistance to radiotherapy and doxorubicin,45-47 but also induces interleukin (IL)-6/STAT3/NF-κB-mediated signalling as well as the PI3K pathway and thereby confers resistance to trastuzumab.56 In line with other tumour-suppressive miRNAs, miR-450b-3p, 520c-3p, miR-520b-5p, and miR-587-5p were shown to increase the effectiveness of trastuzumab.54
microRNAs in Resistance to Endocrine Therapy
Mechanisms of endocrine resistance in breast cancer often involve the loss of ER-α expression, for instance, by hypermethylation of the ER gene which mediates resistance to tamoxifen. Moreover, increased activity of the HER2, IGFR1, or FGFR1 signalling pathways activates MAPK or PI3K signalling and is thereby also able to confer resistance to tamoxifen by sustaining proliferation and anti-apoptotic signals in an ER-independent manner.57 miR-320a is one of the miRNAs which sensitises ER+ breast cancer cells to endocrine therapy. In fact, miR-320a increases the sensitivity of ER+ resistant breast cancer cells to tamoxifen by downregulation of cAMP-regulated phosphoprotein and oestrogen-related receptor gamma.58 miR-451 is another sensitiser to tamoxifen since the miRNA directly targets 14-3-3ζ59 which also leads to increased efficacy of doxorubicin treatment as previously described.20 Overexpression of miR-451 suppressed HER2, EGFR, and MAPK activation and increased apoptosis, leading to an increase in sensitivity to tamoxifen.59 Besides increasing sensitivity to trastuzumab,49 miR-375 has also been shown to enhance the effectiveness of tamoxifen treatment. Knocking down metadherin, a direct target of miR-375, enabled the effect of miRNA re-expression to be partially phenocopied.60 Among the miRNAs with an oncogenic role in endocrine therapy resistance is miR-21. In addition to mediating resistance of breast cancer cells to radiotherapy and doxorubicin as well as HER2+ breast cancers to trastuzumab,45-47,56 miR-21 decreases the sensitivity of ER+ breast cancer cells to tamoxifen by targeting PTEN.61 Other miRNAs confer resistance to tamoxifen target SOCS6 and thereby blocking the SOCS6-STAT3 pathway, such as miR-155,62 or suppress the tumour-suppressor genes PTEN, CDKN1A/p21, and retinoblastoma protein in ER+ breast cancer cells, such as miR-519a.63 Further microRNAs have been shown to act as tumour-suppressors and to sensitise breast cancer cells towards endocrine therapy, such as miR-15a, miR-16, and miR-342-3p, whereas others, for instance, miR-221/222, miR-101, miR-301, and miR-181b, promote resistance to endocrine therapies.64,65
CIRCULATING microRNAS IN THERAPY RESISTANCE OF BREAST CANCER PATIENTS
Several microRNAs can be detected in blood plasma and are therefore known as circulating miRNAs. Their detection in blood samples potentially allows their use as biomarkers, e.g. to predict or monitor therapy response. High levels of miR-210 in the plasma of breast cancer patients, for instance, were shown to correlate with high sensitivity to trastuzumab treatment,66 whereas high levels of circulating miR-125b were associated with an impaired response to the chemotherapeutic drug 5-fluorouracil.67 However, a panel of biomarkers usually provides more reliable information about how a patient might respond to a certain therapy. In the case of miR-19a and miR-205, high circulating levels of both miRNAs predict a bad response of Luminal A patients to neoadjuvant treatment with epirubicin and paclitaxel.68
microRNAS INFLUENCING THERAPY RESISTANCE VIA PLURIPOTENCY, CANCER STEM CELL PROPERTIES, OR EPITHELIAL-MESENCHYMAL-TRANSITION
Since so-called cancer stem cells (CSC) are considered to be tumour-initiating cells and are a major reason for the development of therapy resistance,69 miRNAs regulating stem cell features are of particular interest in understanding the impact of the miRNome on breast cancer therapy resistance. These critical cellular features include pluripotency, developmental pathways which are in charge of the self-renewing potential of stem cells in general, and EMT since breast CSC frequently undergo EMT.70 miR-200c, for instance, targets ZEB-1 as well as Sox2 and Klf4, affecting EMT and pluripotency, respectively.70 Moreover, other miR-200 family members as well as the miR-183 cluster, the miR-221-222 cluster, miR-142, miR-214, let-7, miR-22, and miR-27b have been described to influence the features of CSC by regulating pathways important for stemness and pluripotency, such as the Wnt and Notch pathways, apoptosis, and EMT.71,72
CONCLUSION
In summary, a multitude of miRNAs have been described to sensitise breast cancer cells to chemotherapy, radiotherapy, or targeted therapy or to mediate resistance to diverse treatment strategies. However, the number of miRNAs associated with a tumour-suppressive function exceeds the number of oncomiRs, especially in resistance to chemotherapeutic drugs. This underlines how crucial it is to obtain a better understanding of the complex miRNome and that unravelling further oncomiRs, which could also be explored as potential therapeutic targets, is necessary. Figure 3 summarises the phenotypes and mechanisms by which miR-200c, a tumour-suppressive miRNA, sensitises breast cancer cells to various treatment options. This example teaches us that miRNAs tend to have multiple phenotypes by affecting various cellular mechanisms, it can be expected that in the future, multiple targets of additional studied or uncharacterised miRNAs will have similarly pleiotropic phenotypes.
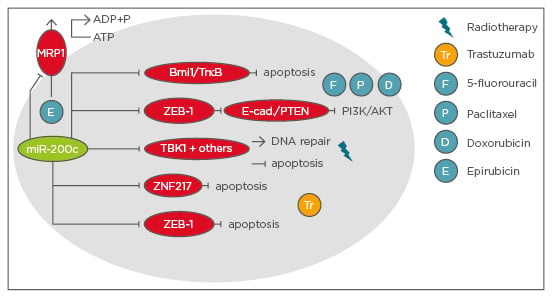
Figure 3: Summary of phenotypes and mechanisms of miR-200c sensitising breast cancer cells to therapy.








